enantiomers
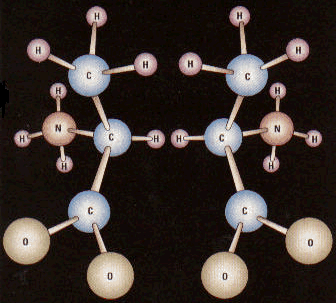
Enantiomers of the amino acid alanine.
Enantiomers are molecules that are optical isomers, or mirror images, of one another. They can be distinguished by the direction in which they rotate the plane of polarization of polarized light and are referred to, therefore, as being dextrorotatory (d-) or levorotatory (l-).
Enantiomers can exist when there is an asymmetric carbon atom within the molecule, i.e., a carbon that is attached to four different structures. The example illustrated is the amino acid alanine, which occurs as l-alanine (left in the picture) and d-alanine (right). Chemical synthesis in the laboratory usually produces equal amounts of the two enantiomers, known as a racemic mixture. However, in living organisms there is a rigid preference for one over the other.
Bias in terrestrial life
All organisms on Earth use amino acids and sugars in only one of the two possible mirror-image forms: the amino-acids are all left-handed (L-) and the sugars are all right-handed (D-). This has prompted theorists to speculate on how such a bias came about. One possibility is that the inorganic molecules which provided a substrate on which prebiotic chemicals assembled in pools on the young Earth were, themselves, of a particular handedness. Another theory is that the choice was initially made in space, by the action of circular polarized light, before even the solar system took shape (see amino acids, in space).
Research, published in 1998, by Keno Soai and his colleagues at the Science University of Tokyo,1 supports the view that once an enantiomer imbalance of even a few percent had arisen it would be amplified by subsequent chemical reactions. Soai's team examined a mixture of compounds containing a slight excess of one enantiomer of the amino acid leucine. The components of the solution reacted to give a substance known as pyrimidyl alkanol, also with a slight excess of one enantiomer. This molecule, however, then acted to catalyze its own formation (see autocatalysis), so that quickly almost all the pyrimidyl alkanol in the solution was of the same handedness. Although pyrimidyl alkanol is not itself relevant to prebiological development, its autocatalyzing behavior shows how a minor enantiomer imbalance could quickly give rise to the bias found in living systems. The fact that a choice was made has proved vital, because proteins (which are made from amino acids) can fold consistently only if made entirely of one form or another, but not both.
Reference
1. Shibata, T., Yamamoto, J., Matsumoto, N., Yonekubo, S., Osani, S., and Soai, K. "Amplification of a Slight Enantiomeric Imbalance in Molecules Based on Asymmetric Autocatalysis: The First Correlation Between High Enantiomeric Imbalance in Chiral Molecules and Circularly Polarized Light," Journal of the Maerican Chemical Society, 120 (46), 12157 (1998).


