borane
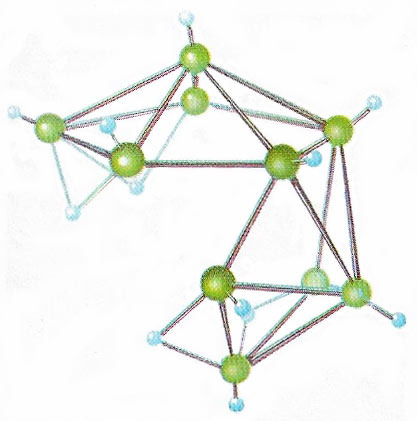
In most covalent bonds two electrons hold two nuclei together. By adopting tightly geometric patterns electron-deficient compounds, such as this deca-borane, can hold together with a smaller number of electrons.
Borane is a covalent hydride of boron, of unusual molecular structure. Boranes have hydrogen-bridge bonding, and the boron atoms form the vertices of polyhedra. Boranes are volatile, reactive, and often flammable in air. They may be used as high-energy fuels for rockets and jet planes.
The simplest borane is B2H6, made by treating magnesium boride with acid. The higher boranes have up to 10 boron atoms.


