chemical reaction
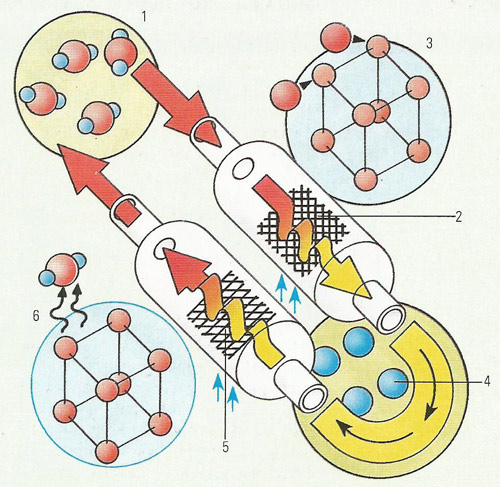
An example of a reversible chemical reaction. If steam (1) is passed over iron that is being heated in an enclosed tube (2), a reaction will take place producing iron oxide (rust) (3) and hydrogen (4). If hydrogen is passed over rust and heated (5), water and iron are formed (6).
A chemical reaction is a change or process in which chemical substances convert into other substances. This usually involves the breaking and formation of chemical bonds. Reaction mechanisms include endothermic, exothermic, addition, condensation, combination (formation of a compound), decomposition, oxidation-reduction (see redox) reactions.
A reversible reaction is one in which the products can change back into the reactants. Thus nitrogen and hydrogen can be combined to give ammonia (see Haber process) and ammonia may be decomposed to nitrogen and hydrogen. Such processes yield an equilibrium mixture of reactants and products.


