ionic bond
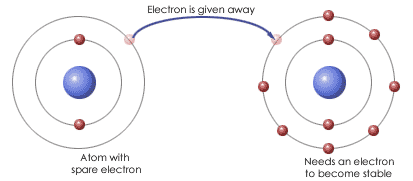
The formation of an ionic bond.
An ionic bond is an attraction between ions of opposite charge in an ionic compound. Such bonds, which result from the transfer of electrons, are not formed between particular ions but exist between an ion and all of the oppositely charged ions in its immediate vicinity.
Very few materials have bonds that at truly ionic; most ionic bonds are, at least, partially covalent. The fraction of ionic character of a bond, f, can be estimated from the electronegativities (X) of the two atoms according to the formula:
![]()
Ionic compounds
Among ionic compounds are acids, bases and salts. Examples include magnesium oxide (MgO) and sodium fluoride (NaF). As crystalline solids, with negative and positive ions arranged alternately in the lattice, such compounds have high melting points above 250°C (480°F) and boiling points above 500°C (930°F). They are usually soluable in water but insoluble in organic solvents. As solids, ionic compounds are nonconductors of electricity, but in solution and in their molten state, they are conductors.


