mechanical equivalent of heat
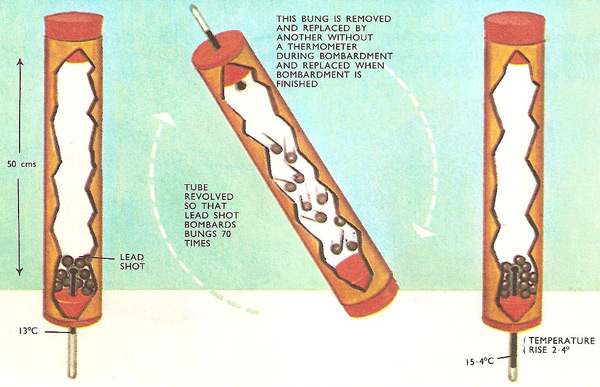
Mechanical work done = total distance through which
shot has fallen × mass of shot × acceleration
= 0.5 × 70 × mass × 9.81 joules
Heat generated = mass of shot × specific heat of lead ×
temperature rise
= mass × 0.0315 × 2.4 calories
Work equivalent to 1 calorie = (0.5 × 70 × mass × 9.81)/(mass
× 0.0315 × 2.4) = 4.5 joules
The result is rather high because some of the heat is lost to the
cardboard of the tube.

As a space capsule returns to Earth, friction with the atmosphere creates a great deal of heat. Mechanical energy is converted to heat energy. The heat shield prevents the metal of the capsule from becoming hot enough to melt.
After vigorously sawing through an obstinate log of wood, the blade of the saw usually becomes too hot to touch. Obviously heat has been generated, but how? The heat must have come from somewhere. It cannot have sprung from the blade itself because this was at quite a low temperature to start with. Nor has the heat come from the wood, for the wood has undergone no drop in temperature.
Energy has been used up in sawing. Most of this energy is used up in overcoming friction with the rough wood. Now this energy cannot just disappear. If it disappears in one form it must appear again in another. The energy used to overcome friction appears again as heat and the temperature of the saw blade rises.
In other words, mechanical energy is converted into heat energy. The human body uses this fact. In bitterly cold weather people try to keep warm by rubbing their hands together and jumping up and down.
The mechanical energy put into rubbing hands is used in overcoming friction and heat is produced. Mechanical energy is also used in jumping. As the body rises higher and higher, this energy is stored as potential energy (energy stored ready for use). As the body descends and gathers speed it changes into kinetic energy (energy by virtue of motion). But once back on the ground again, the movement is halted. There can be no more kinetic energy or potential energy. It has all changed into heat. This happens when any falling body plummets onto the ground.
Mechanical energy and heat energy are interchangeable. 4.2 million joules of mechanical energy are expended in producing 1 calorie of heat. This value, 4.2 joules per calorie, is known as the mechanical equivalent of heat. One calorie is the quantity of heat needed to raise the temperature of 1 gram of water through 1°C. A joule is the work done on a mass of 1 kilogram to make it move through 1 meter with an acceleration of 1 meter per second per second (1m/s2)
Very little apparatus is needed to get a rough value of the mechanical equivalent of heat experimentally – just a long cardboard tube with three rubber bungs to fit tightly into the ends, a centigrade thermometer, and about a pound of lead shot. One of the rubber bungs is pierced to hold a thermometer.
The idea is to put the lead shot in the tube and rapidly tilt the tube so that the shot tumbles back and forth bombarding the rubber bungs at the ends. Each time the shot falls it loses energy of motion and gains heat. The temperature goes up. The numerical value of the mechanical equivalent of heat can be calculated, from the number of times the lead shot bombards the bungs, the length through which it falls, and the temperature rise.


