Diels-Alder reaction
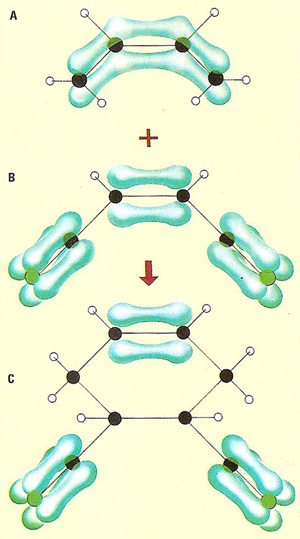
A Diels-Alder reaction depicted using molecular orbitals. See main text for description and key.
A Diels-Alder reaction, also known as diene synthesis, is a reaction discovered by Otto Diels and Kurt Alder, which is important in many chemical syntheses, including those involved in making some plastics, insecticides, and fungicides.
A conjugated diene, such as 1,3-butadiene isoprene, adds readily to a "dienophile" containing a double or triple bonded activated by an adjacent nucleophile group.
The Diels-Alder reaction has served an important role in many syntheses because it smoothly and stereospecifically unites two carbon compounds. A six-membered ring is formed by 1,4-addition of an alkene unit to a conjugated diene. The alkene unit (known as the dienophile) has electron-withdrawing groups which activate the diene.
The upper illustration here shows a Diels-Alder reaction using molecular orbitals. The addition reaction is shown by the rearrangement of the electron clouds.
Key (to upper illustration)
A) Butadiene. This is a conjugated diene (two double bonds separated by a single bond). It is shown is the s-cis form and has the property of undergoing 1.4 addition very easily.
B) 1,2-dintrile ethene. This is the dieneophile, and the electron-withdrawing or accelerating groups are nitrile groups. It is these that activate diene.
C) The diene and dienophile react to form 2,3-dintrile benzene. The configurations of the diene and dienophile are retained in the product, which means that the reactants come together to give cis addition.


