evaporite
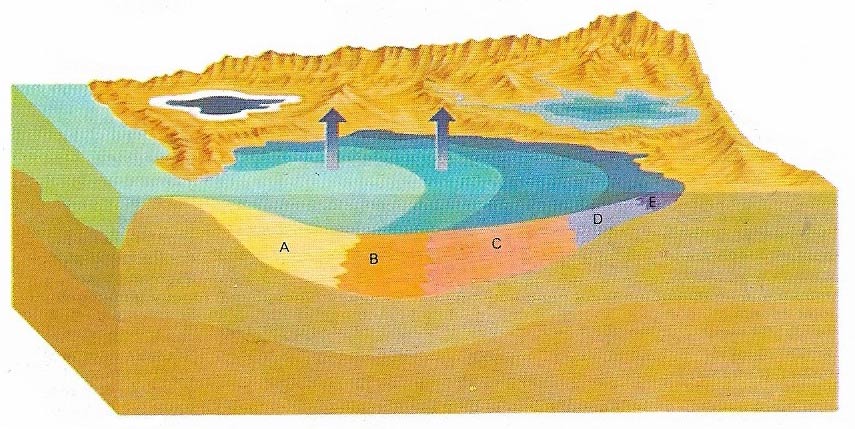
The formation of evaporites takes place in dry, arid regions. Seawater flows into the gulfs where it tends to evaporate, concentrating the dissolved salts and making the water very saline. Finally, the salts are precipitated out when the solution becomes too concentrated to hold them. When subsidence occurs at the same time as the precipitation of the salts, very thick deposits are formed. When half a seawater sample has evaporated, calcium and magnesium carbonates (A) precipitate out. They are completely removed from the brine when it is 15% of its original volume. At about 20% calcium sulfate (B) starts precipitating, followed by sodium chloride or common salt (C), other sulfates (D), rare salts of magnesium, potassium, sodium and borates anf fluorides (E). Finally, magnesium and potassium chloride precipitate (F).
An evaporite is a sedimentary deposit of salts that have been precipitated from solution owing to the evaporation of a body of water. Evaporite deposits have the least soluble salts at the bottom (calcium salts), followed by the very soluble halite (common salt) and magnesium and potassium salts. Most important commercially are gypsum (CaSO4.2H2O), anhydrite (CaSO4), and halite (NaCl).
Alkali flats
Alkali flats, also known as salt flats, are level, barren areas in dry regions covered with evaporites, mainly salts of the alkali metals and alkaline earth metals. Alkali flats are formed by the repeated periodic evaporation of shallow lakes lacking outlets.


