fatty acids
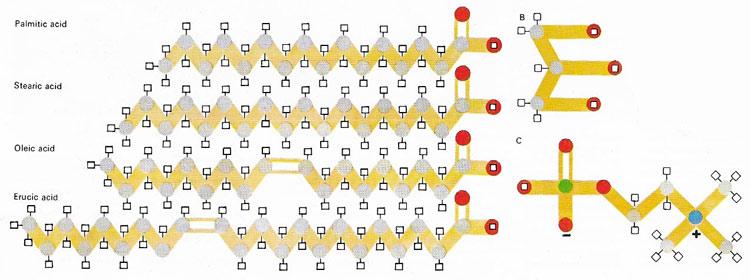
Fatty acids are organic molecules that all have a carboxyl group (–COOH) at one end of a chain of carbon atoms that may vary in length and in the number of hydrogen atoms attached to each carbon. They are a fundamental component of lipids, compounds found in materials as diverse as bacon fat and olive oil. In the most common lipids, three fatty acid molecules are linked chemically to one molecule of glycerol (B). In some important biological molecules, one of the fatty acids may be replaced by a phosphorus-containing molecule, such as choline phosphate (C), to form a type of compound known as a phospholipid.
Fatty acids are organic acids consisting of long, straight hydrocarbon chains which terminate in a carboxyl group (-COOH) and have an even number of carbon atoms. Many fatty acids occur in living things as components of lipids, including triglycerides. Some fatty acids can be synthesized by the body; others, the essential fatty acids, must be obtained from the diet.
Natural fatty acids commonly have a chain of 4 to 28 carbons (usually unbranched and even-numbered), which may be saturated or unsaturated. The most important of saturated fatty acids are butyric (C4), lauric (C12), palmitic (C16), and stearic (C18). The most common unsaturated acids are oleic, linoleic, and linolenic (all C18).
The physical properties of fatty acids are determined by chain length, degree of unsaturation, and chain branching. Short-chain acids are pungent liquids, soluble in water. As chain length increases, melting points are raised and water-solubility decreases. Unsaturation and chain branching tend to lower melting points.
Acid number, also called acid value, is the number of milligrams of potassium hydroxide needed to neutralize the free fatty acids in one gram of a specified substance. The acid number is used to measure the fatty acid contents of fats, oils, etc.


