gemstone
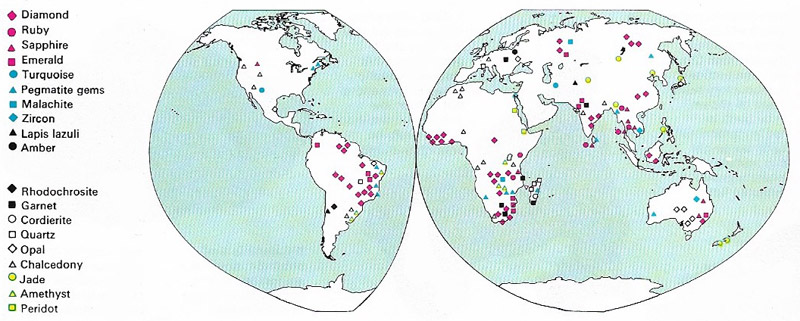
Figure 1. The gem-producing regions of the world are shown on the map. The diamond, ruby, sapphire, and emerald are classed as precious stones; all other gems are classed semi-precious stones.

Figure 2. The Kimberley Great Hole is a disused diamond mine 300 meters (1,000 feet) deep. Diamonds were found at Kimberley in 1871 and some three tonnes of gems were removed before work ended in 1909.
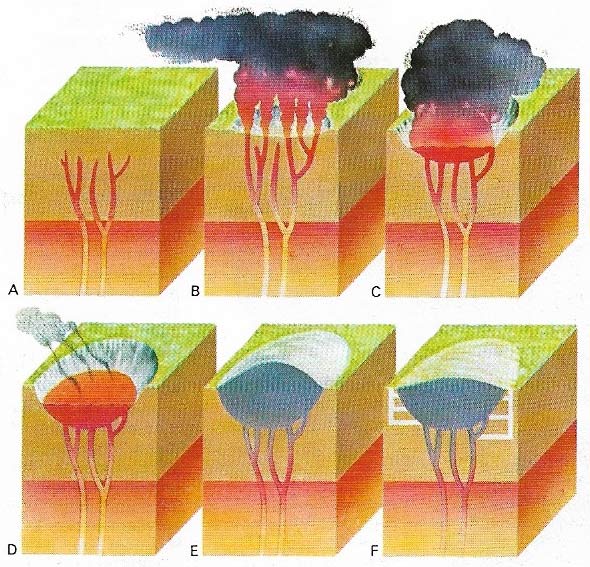
Figure 3. Diamonds are made by intense heat and pressure in volcanic plugs or kimberlite pipes, deep below the Earth's crust (A). As pressure increases gas collects in fissures and explodes, forming a hollow in the Earth's surface (B). Diamond-bearing kimberlite wells up the fissures (C) to fill the cavity (D). The kimberlite may rise above the surface (E). Shafts are sunk for easier access to it (F).
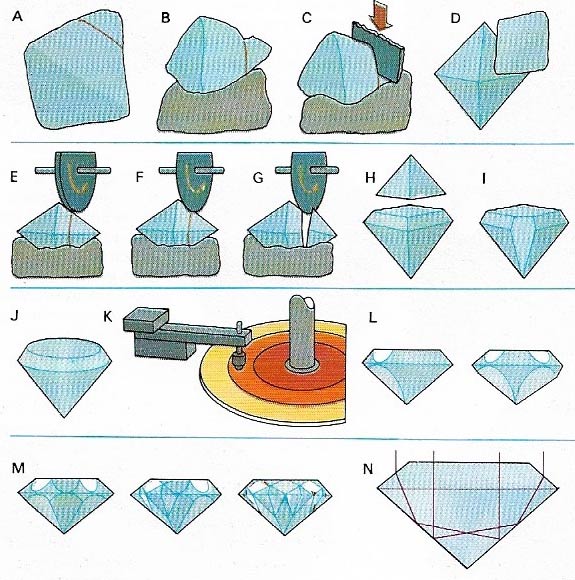
Figure 4. A rough diamond is cut to shape along the cleavage planes (A). These are first grooved (B) and then cleaved with a blade (C) until a workable shape (D) is obtained. C utting is done first with a coarse (E) and then a fine (F) saw to give the final shape (G, H, I, J). Polishing is done on a lap (K), covered in diamond powder, producing the facets (L, M) that reflect light from the interior giving the stone its fire (N).
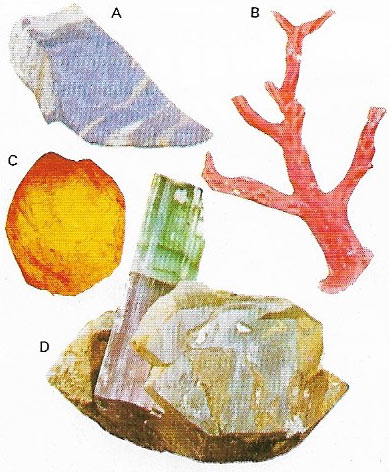
Figure 5. Most gems are mineral. Lapis lazul (A) is the name given to a rock rich in lazurite. Tourmaline (D) is a complex borosilicate. Organic gems include coral (B) (the skeletons of coral polyps) and amber (C) which is a fossil resin.
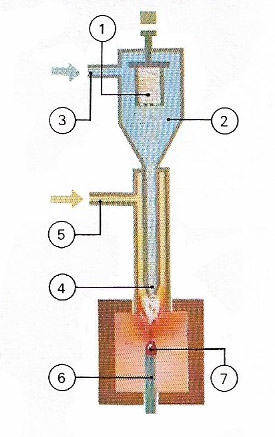
Figure 6. Synthetic gems are produced by a flame-fusion process, known as Verneuil's method, after the inventor. Alumina powder in the container (1) falls into the chamber (2). Oxygen (3) mixes with the alumina and carries it to the tip of the torch (4) where it burns with hydrogen entering through the tube (5). In the intense heat produced, the fine alumina particles fuse into gem droplets and fall onto a support (6) on which the body of the gem is being formed (7).

Figure 7. Topaz. Credit: MII.
A gemstone is stone prized for its beauty, and durable enough to be used in jewelry and for ornament. A few gemstones – amber, coral, pearl, and jet – have organic origin, but most are well crystallized minerals. Transparent stones, such as diamond, ruby, emerald, and sapphire, are the most highly valued. Fashion often determines the popularity of gems. Jet, a hard, shiny black fossilized wood, was widely used in jewelry in the years after 1861, when Queen Victoria was in mourning.
Formation of gems
Gems are that are of inorganic origin and occur as natural minerals are formed in several different ways. Many gems are found in igneous rock, that is rock formed directly from magma that has welled up from the Earth's interior and solidified beneath the surface. As magma cools, the elements tend to separate into regions where they form different minerals. Pockets of gases and superheated water often dissolve many elements; these finally cool and combine to form precious or semi-precious minerals. Pegmatite – light-colored, coarse-grained igneous rocks – are formed by superheated gas and water and they often contain stocks of gems including beryl, quartz, tourmaline, and feldspar. Gases in the cooling pegmatites help form minerals such as fluorine-bearing topaz (Figure 7) and tourmaline (Figure 19).
Other gems are formed when heat, pressure, or chemical action alter the structure of existing rocks causing them to recrystallize or re-form in different ways as metamorphic rocks. Olivine (also common in lavas), emerald (Figure 11), and garnet (Figure 12) are found in metamorphic rocks. Great heat and pressure were probably responsible for diamond formation from carbon in kimberlite (Figure 3). Many gem materials, including diamond (Figure 10), are found as crystals or rolled pebbles in alluvial gravels of river beds.
Properties of gems
Gem minerals can be identified by a number of qualities such as the shape of the uncut crystals, their color, hardness, refractive index, and specific gravity (density). A gem's value is established by rarity, brilliance, purity, color, and hardness. Demand also determines the value of a gem; diamonds are in constant demand for industrial use as cutting instruments as well as for jewelry.
One of the main factors that determines the beauty of a gem is its properties with regard to light – namely, how light is reflected, refracted (bent), and dispersed (split up) into the spectrum by the gem. Each stone has its own refractive index. This index is measured by dividing the sine of the angle of incidence (the angle between the ray of light and the normal, which is a line drawn perpendicular to the surface) by the sine of the angle of refraction. Of all natural gems, diamond has the highest refractive index. Its ability to disperse white light gives the diamond its special fire, or flashes of spectral color and characteristic brilliance.
The colors of diamonds are generally due to lattice defects in the crystal and rarely to trace elements. Most other gemstones are colored by metal oxides that are either impurities or components. Color, however, is the feature that gives many gems their special quality. The transparent red ruby (Figure 16) and the blue sapphire (Figure 17), both of which are forms of a normally dull, gray or colorless mineral called corundum, the green emerald, a form of the mineral beryl (Figure 11), ad the yellow topaz are all admired because of their pure tints. Opaque or cloudy gems such as opals (Figure 13) depend entirely upon their color to make them attractive.
Specific gravity is the weight of the mineral compared with the weight of an equal amount of water. Diamond, for example, has a specific gravity of 3.52, which means that it weighs 3.52 times as much as an equal amount of water, whereas amber has a specific gravity of 1.07. The weight of a diamond is usually expressed in carats – one carat is equivalent to 200 milligrams.
Hardness ensures durability and accordingly the most valuable gems are stones that will wear for a long time. Hardness is measured on the Mohs scale, which consists of numbers fro one to ten, indicating the relative hardness of substances. Diamond has a hardness of 10 on this scale and is by far the hardest of all natural substances. It is about 90 times harder than corundum, which rates as nine of the scale. Some gems are quite soft and are valued for other properties, including chatoyancy (cat's-eyes), dichroism (see double refraction), opalescence, and asterism – a star-shaped gleam caused by regular intrusions in the crystal lattice.
Polishing and cutting of gems
Since earliest times gems have been engraved in intaglio (where the design is cut into the stone) and cameo (where the design is in relief). In the 15th century, notably in Italy, the cutting technique of faceting was developed. The oldest form in which gems were cut was a rounded shape called cabochon, a French word for head. The cabochon is used for stones showing the effects of chatoyancy and asterism, which are caused by reflections from inclusions. Later, other styles of cut, including the brilliant, step, and mixed cuts, were developed. These depend on various facets being ground and polished in symmetrical arrangements to remove surface flaws and heighten the color or brilliance of the stone (Figure 4). Some gems are dyed, impregnated, heated, or irradiated to improve their color. Synthetic gems are made by flame-fusion or by crystallization from a melt or aqueous solution (Figure 6).
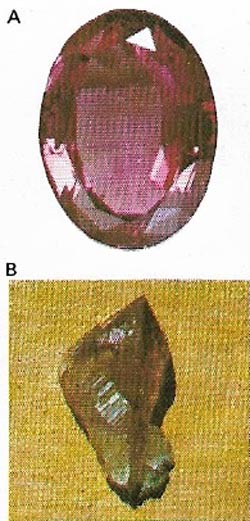 |
| Figure 8. Amethysts (A) are a form of transparent quartz (B), with a violet or purple color. They are mined in Russia and South America. |
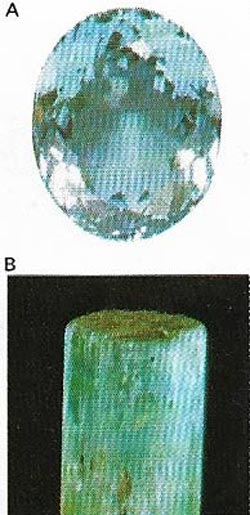 |
| Fig 9. Aquamarines (A) are a blue or blue-green variety of the mineral beryl (B). The best are mined in Brazil and the Urals. |
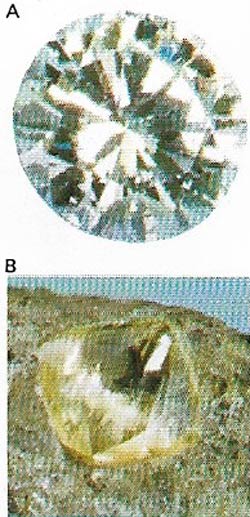 |
| Figure 10. Diamonds (A) are pure crystallized carbon (B), the hardest natural substance. South Africa is the main source. The most prized are colorless. |
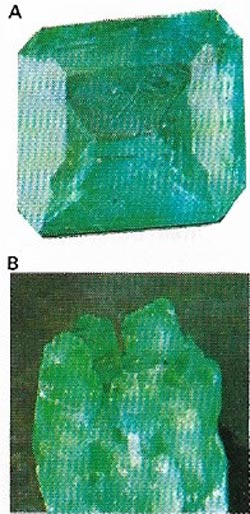 |
| Figure 11. Emerald (A) is a gem-quality, rare green variety of the mineral beryl (B). The best emeralds occur in Columbia in South America. |
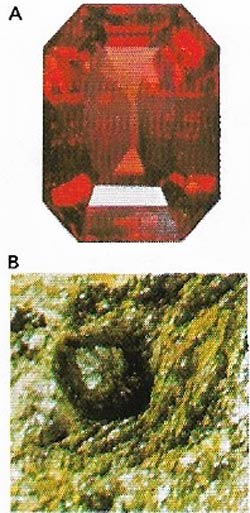 |
| Figure 12. Garnets (A) are formed from silica and two metals (B). Those with aluminum and magnesium are the prized ruby-red pyrope. |
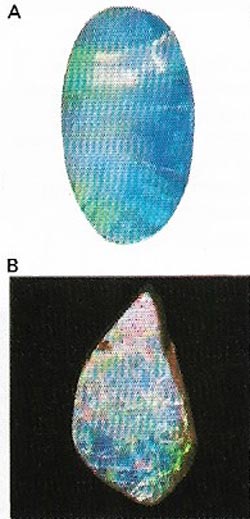 |
| Figure 13. Opals (A) are a form of hydrated silica (B). The most prized specimens are the so-called black opals found in Australia, which flashes of several iridescent colors. |
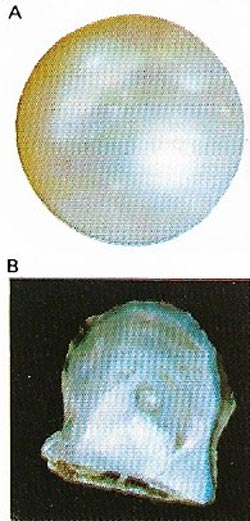 |
| Figure 14. Pearls (A) are organic gems produced mainly by oysters (B) from nacre, the iridescent substance forming the inner layer of the shell. Pearls are prized for their luster. |
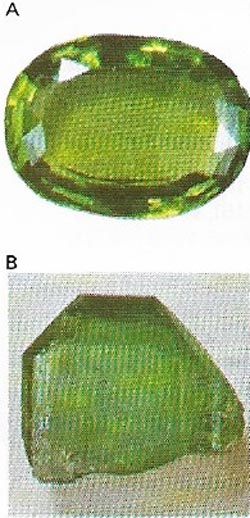 |
| Figure 15. Peridots (A) are a transparent green variety of the mineral olivine (B), which is a magnesium-iron silicate. The finest come from Burma and Thailand. |
 |
| Figure 16. Ruby (A) is a red variety of the hard gray or colorless aluminum oxide mineral, corundum (B). The finest rubies, from Burma, are colored a deep bluish-red by a chromium impurity. |
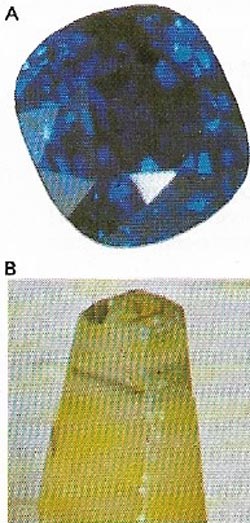 |
| Figure 17. Sapphire (A), like ruby, is a variety of the mineral corundum (B). It occurs in many colors but the most valued are blue. The best kinds come from Murma and Thailand. |
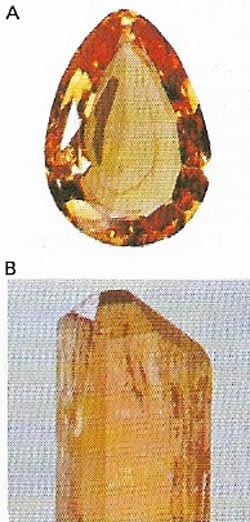 |
| Figure 18. Topaz (A) is a mineral compound of aluminum, silica, and fluorine (B). The most prized varieties are yellow. It is found mainly in Brazil, Russia, and the United States. |
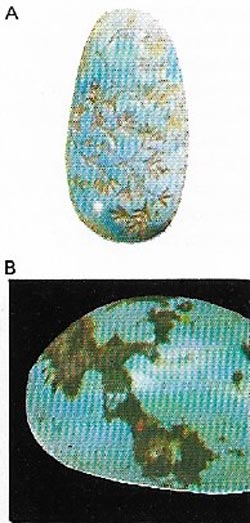 |
| Figure 19. Turquoise (A) is a hydrous copper-aluminum phosphate (B) sometimes containing iron. The most prized color is sky blue and comes from Iran. Its name means Turkish stone. |


