tritium
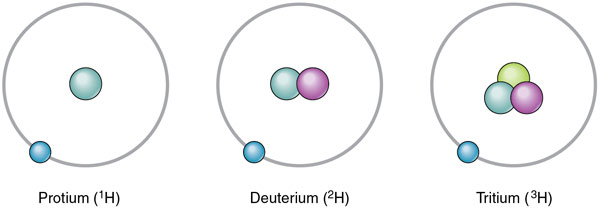
The three isotopes of hydrogen.
Tritium is the heaviest of the three isotopes of hydrogen (T or 1H 3), in which each nucleus contains one proton and two neutrons, instead of only one proton as in normal hydrogen or one proton and one neutron as in deuterium. The nucleus of tritium is sometimes called a triton. Tritium (from the Greek tritos, meaning "third") is radioactive, with a half-life of 12.323 years. It decays by beta emission. Only one atom in 1017 of natural hydrogen is tritium.
Tritium is readily produced in nuclear reactors and is used in the production of the hydrogen (fusion) bomb. It is also used (along with deuterium) in experimental nuclear fusion reactors and as a radioactive agent in making luminous paints, and as a tracer.
Tritium was named by Urey and Murphy in 1933, a year prior to its discovery. Since about 1942 the tritium nucleus has been called the triton.
| relative atomic mass | 3.0 |
| melting point | -252.5°C |
| boiling point | -248.1°C |


