neutron
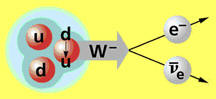
Neutron decay.
The neutron is an electrically neutral subatomic particle that, together with the proton, is found in the nucleus of atoms. It has a mass of 939.6 MeV – 1838.65 times that of the electron and marginally more than the mass of the proton.
Because it is electrically neutral, the neutron must be bound into the atomic nucleus by a powerful force that does not depend on electric charge; this is the so-called strong force (or strong nuclear force). Within a nucleus the neutron is stable, but in a free state is unstable with a half-life of 885±0.7 seconds (about 15 minutes), decaying into a proton, an electron, and an anti-neutrino:
![]()
This slow decay of the neutron is indicative of yet another nuclear force, called the weak force.
Despite being electrically neutral, the neutron does have both an electric dipole moment (as if it were made of a positive and a negative electric charge separated by a minute distance) and a magnetic moment, both of which are indicative of some internal electrical structure. Indeed, it is now known that the neutron (like the proton and other baryons) is composed of one up quark and two down quarks.
Neutrons are highly penetrating, and are moderated (slowed down) by colliding with the nuclei of light atoms. They induce certain heavy nuclei to undergo nuclear fission. Shielding requires thick concrete walls.
Neutrons are detected by counting the ionizing particles or gamma rays produced when they react with nuclei. Neutrons have wave properties and their diffraction is used to study crystal structures and magnetic properties.
Discovery of the neutron
In 1930, Walther Bothe and Herbert Becker discovered that when beryllium is bombarded with alpha particles, it emits a very penetrating radiation. They assumed that it must be akin to gamma rays as, like them, it could pass through several inches of lead. Irene Curie and F. Joliot showed that these beryllium rays could knock particles out of paraffin wax with extraordinary force.
The particles proved to be protons moving with very high energy. A simple calculation showed that if the beryllium radiation had caused the acceleration, then its energy must be about 50 million electron-volts, which then appeared as an enormous energy.
The publication of this remarkable research attracted the attention of James Chadwick, a colleague of Ernest Rutherford. Rutherford had long before visualized the existence of a neutral particle, which (in 1921) he called the neutron, and more than a decade before had forecast its main properties. Chadwick had assisted Rutherford in attempts to discover the neutron, but they had not been successful. Chadwick saw that the discovery of the Joliot-Curies could be explained if the beryllium radiation consisted, not of wave-radiations, but of neutral particles. He quickly performed the confirmatory experiments, and announced the discovery of the neutron; a neutral particle slightly heavier than the positively-charged proton.
The neutron was the fourth of the fundamental particles to be discovered. Its discovery gave tremendous impetus both to experimental and theoretical research. A few weeks later in 1932, J. D. Cockcroft and E. T. S. Walton announced the first successful disintegration of atoms by machinery. They accelerated protons to a sufficient energy to disintegrate atoms of lithium. This was the beginning of the controlled entry into the nucleus of the atom.
Prompt and delayed neutrons
A prompt neutron is a neutron resulting from nuclear fission (either during the fission process or from freshly formed fission fragments) which is emitted without measurable delay, i.e. less than a millionth of a sec. A delayed neutron, by contrast, is emitted following fission which with a measurable time delay. Only a small proportion of neutrons are delayed, but the average delay period must be taken into account in the control of nuclear reactor.
Fast and thermal neutrons
A fast neutron is a neutron resulting from nuclear fission that has lost little of its energy by collision and therefore travels at high speed. It is usual to describe neutrons with energies in excess of 0.1 MeV (see electronvolt) as "fast". However, fission induced by fast neutrons is often described as "fast fission" and in this context the neutrons are so described if they have energies in excess of the fission threshold of uranium-238, i.e. above 1.5 MeV.
A thermal neutron is a neutron of very slow speed and consequently of low energy. Their energy is of the same order as the thermal energy of the atoms or molecules of the substance through which they are passing; i.e. about 0.025 electronvolts, which is equivalent to an average velocity of about 2,200 meters per second. Thermal neutrons are responsible for numerous types of nuclear reactions, including nuclear fission.
Neutron activation analysis
Neutron activation analysis is a highly sensitive method of identifying the chemical composition of a substance. The sample is bombarded with high-energy neutrons that are then absorbed by the atoms present. The resulting radioactive nuclei emit radiation of an energy and decay rate characteristic of the original atoms, which can thus be identified. The quantity present can also be found with extreme precision.

