Rutherford, Ernest (1871–1937)
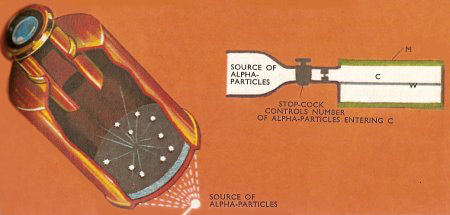
An alpha particle may be detected when it falls on a zinc sulfide screen, because it produces a flash of light which may be observed through an eyepiece (left). This method was used by William Crookes. Rutherford and Hans Geiger devised an electrical method of detecting single alpha particles. In their apparatus (right), alpha particles were let, a few each second, into the chamber C. In this chamber, the metal cylinder M surrounded the insulated wire W, and a high-voltage difference was applied between M and W. When an alpha particle collided with a gas molecule in C, the molecule lost an electron, and this electron was accelerated in the electric field and collided with a second gas molecule when another electron was set free. The whole process was repeated many times in succession. In this way an electron avalanche was built up, which could be detected as an electrical impulse.
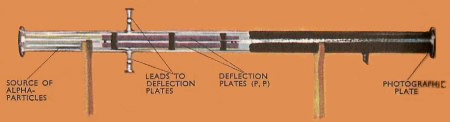
The apparatus that Rutherford used to determine the ratio of electric charge to mass of he alpha particle. The deflection of the particles in the electric field between PP was compared with the deflection in a magnetic field, and the required ration was obtained from the magnitudes of these deflections.
Ernest Rutherford was a brilliant experimental physicist and one of the founders of nuclear physics. He was born in Brightwater, New Zealand, into a family of pioneer stock who had emigrated from Britain less than 30 years earlier. Although he was a very good all-round scholar while at school, Rutherford showed no real bias to science. In 1890 he entered Canterbury College at Christchurch in New Zealand, where his scientific ability became apparent, and graduated with first-class degrees in both science and mathematics.
 |
He continued at Christchurch doing research work, and developed a detector for radio waves which depended on the magnetization of iron. In 1894 a scholarship was offered by Cambridge University to a postgraduate student in New Zealand, and Rutherford won it. Borrowing the money for his boat fare, he left his family for England in 1895.
At Cambridge, J. J. Thomson was the professor at the famous Cavendish Laboratory, and he was at this time working on experiments into gases which conduct after exposure to the recently-discovered X-rays. Rutherford stopped work on his radio-wave detector, which eventually Marconi took up and from which was eventually established the potentiality of radio broadcasts, to join J. J. Thomson in his work.
Even as Rutherford helped J. J. Thomson in his experiments in 1896, the discovery of radioactivity was announced by Henri Becquerel in Paris. In 1897, J. J. Thomson announced his proof of the existence of the electron, but Rutherford was already working on experiments on gases which conduct after exposure to radioactivity. During this year he noted many of the properties of radioactivity, and became acquainted with the experimental methods in this new science.
In 1898, Rutherford applied for a research professorship at McGill University in Montreal, Canada. Thus, still under 28 years of age, Ernest Rutherford became a professor and entered the first great period of scientific discovery in his life.
In 1901 the English chemist Frederick Soddy helped Rutherford with his work on radioactivity, and within about a year they had realized that a radioactive atom changes to a different atom on emission of radiation. Together they published papers on the cause and nature of radioactivity, and on the "spontaneous transformation" theory of radioactivity. Rutherford also managed to work on alpha particles (beta particles were, by then, known to be electrons), and published a general paper on radioactive change in 1903. In 1904 his first book, Radioactivity, was published.
Within five years Rutherford, with the help of Soddy, had solved many problems of the new subject of radioactivity, had realized that radioactive atoms change spontaneously to other atoms, and had defined the idea of isotopes.
In 1906 the chair at Manchester University was offered to Rutherford by the professor then holding it, who promised to retire if Rutherford would take it. This Rutherford did, and in 1907 he moved to Manchester's new, well-equipped laboratories. Now began his second period of discovery, probably the greatest, and certainly the happiest, of his life.
In 1908, Rutherford received the Nobel Prize in Chemistry for his work on radioactivity, but was now investigating the properties of alpha particles with the help of some of his 15 research students. A series of experiments led him to believe that alpha particles were, in fact, helium atoms, and to prove this in 1909 he performed a simple and clever experiment. A substance (radon) which emitted alpha particles was placed outside a closed evacuated tube having a metal electrode at each end. After a few days, a high voltage was applied between the metal electrodes, and the spectrum of the electric discharge which took place clearly proved the existence of helium atoms in the tube. The alpha particles must have traveled through the walls of the tube and collected, and the alpha particles must be helium atoms. (In fact, we now know that alpha particles are helium nuclei.)
In 1911, Rutherford proposed his most revolutionary idea, concerning the existence of the atomic nucleus. (See Rutherford's experiment and atomic model.) Up to this time the atom was thought of as being a positively charged sphere with negatively charged electrons moving about inside. Two of Rutherford's workers found that several alpha particles bounced back from a thin foil of metal when many alpha particles were allowed to hit the foil. To explain this, Rutherford assumed that the entire positive charge of the atom is concentrated in a very small nucleus, and that the electrons occupy the space outside the nucleus.
This was a revolutionary idea in 1911, though today it is totally accepted: it has been described as the greatest change in our idea of matter since the time of the ancient Greeks. This nuclear theory provided the basis for the new science of nuclear physics.
In 1912, Niels Bohr, the great Danish scientist, joined Rutherford and put forward the Bohr model of the atom, thus obtaining general acceptance of the nuclear atom. In 1914 the Great War began and the scientists dispersed from the laboratory. After a period away from Manchester Rutherford returned to academic research in his empty laboratory, thus entering his third period of discovery. By 1918 he was certain that he had an experiment involving the artificial transmutation of nitrogen (i.e. changing the nitrogen atom to a different atom.)
 |
| The apparatus with which Rutherford first observed
artificial transmutation in 1919. In this equipment, nitrogen atoms
were converted into oxygen atoms, when in collision with alpha particles
from a source in inside the horizontal enclosed tube. Protons ejected
by nitrogen when forming oxygen were detected at the rectangular window
at the end of the tube. Credit: Science Museum, London
|
By 1919, Rutherford had become Cavendish professor at Cambridge, and he rapidly confirmed the artificial transmutation of nitrogen. Alpha particles from polonium were allowed to pass through nitrogen gas; when one struck a nitrogen nucleus, a hydrogen nucleus was ejected, and an oxygen nucleus formed.
In 1920 he named the hydrogen nucleus a proton, and although he did a great deal of research work, he found himself getting more involved in the administration and organization of scientific research. The Cavendish grew in size and reputation under his leadership, but the discoveries were now made by the gifted people under Rutherford aided by his interest and enthusiasm.
Ernest Rutherford was president of the Royal Society from 1925 to 1930, and in 1931 his greatest achievement was recognized when he became Baron Rutherford of Nelson. Still very active and enthusiastic in his scientific interests, Lord Rutherford died after a brief illness in October 1937, and was buried in Westminster Abbey.

