BETWEEN FIRE AND ICE: The Science of Heat - 4. Passing the Heat Along

Figure 1. Spoon in a mug of coffee.
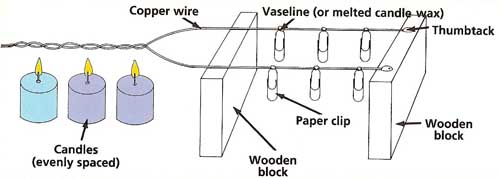
Figure 2. An experiment with conductors.
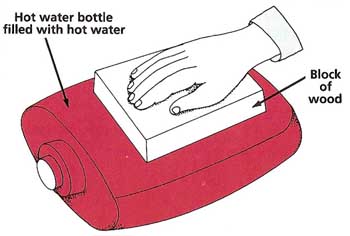
Figure 3. Insulation experiment.
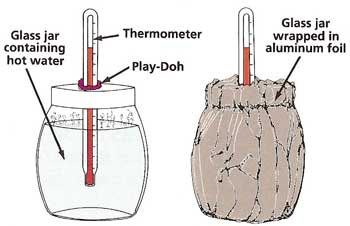
Figure 4. Thermal wrappings experiment.
Leave a metal spoon in a cup of coffee and quite soon the handle of the spoon will become hot. This is an example of heat traveling by CONDUCTION (see Figure 1).
We can understand why the end of the spoon gets hot by once again thinking about molecules.
The fast-moving molecules of water in the coffee bump into the part of the spoon that is next to the hot liquid. This makes the metal molecules of the spoon vibrate harder so that the metal heats up. Quickly the vibrations are passed on, from molecule to molecule, up the handle of the spoon.

A Test of ConductorsYou will need:
What to do:
Taking it further:
You may also wish to try comparing two wires of the same metal but of different thickness. Does heat travel faster along a thinner wire of copper than a thicker one? If so, how much faster? For instance, if one wire is half as thick as another, does heat travel twice as fast along it?
Try making up you own questions and then devising an experiment to answer them. You may find, as scientists often do, that while one experiment may help solve a particular problem, it leads to other interesting problems and further ideas for experiments. |
Conductors and Insulators
Substances through which heat can travel easily are said to be good conductors. Metals are the best conductors. That is why we use them for making such things as saucepans and radiators. It also explains why metals usually feel cool to the touch. If a metal object is at a lower temperature than your body, it will quickly carry heat away from your skin when you touch it. The opposite is true if the metal is warmer than you are. Then the metal will quickly give up its heat to your skin so that it fells hot to the touch.
A substance through which heat cannot travel well is called an INSULATOR. Air, for example, is a very good insulator.

Can the Heat Get ThroughYou will need:
What to do: Fill the hot-water bottle with hot (but not boiling) water and lay it on a table. Place the piece of wood on the bottle and leave it there for 5 minutes. Put your hand on top of the wood. Record whether it feels "very warm," "quite warm," "warm," or "cool." Repeat this with the plastic, paper, and other substances (see Figure 3). From your results, make a list of the materials, with the best conductors at the top and the best insulators at the bottom. You may occasionally need to replace the hot water so that the hot-water bottle is at more or less the same temperature throughout the experiment. |
Protecting the Space Shuttle
 |
As the orbiter of the space shuttle returns to Earth, it plunges through the upper atmosphere at very high speed. Without proper protection this would cause the metal hull of the craft to melt. Scientists have therefore developed a special insulating material, containing silicon, which can be made into thin, lightweight tiles. These tiles are used to cover the orbiter's underside, nose, and wing tips.
A space shuttle tile is such a good insulator that one side can be held in bare hands while the opposite side is red-hot. It can withstand temperatures of up to 2,300°F (1,260°C).

Thermal WrappingsYou will need:
What to do: Ask an adult to make a hole in the middle of the metal lid of the jar, just big enough for one of the thermometers to fit through. Push the thermometer through the hole and seal any gap with some Play-Doh. Wrap the sides and base of the jar with aluminum foil, leaving enough foil free at the top to cover the lid. Fill the jug with hot water from a hot-water faucet (or ask an adult to heat up some water for you). Measure the temperature of this water with the second thermometer (see Figure 4). Immediately fill the jar with hot water from the jug. Put on the lid, cover the lid with foil, and start the stopwatch. After 10 minutes, take off the foil wrapping and record the thermometer reading. Throw out the water and wrap the jar with a different material. Fill the jug again with fresh hot water and make sure that this is at the same temperature as before. Repeat the experiment as with the foil. Do the same with the other wrapping materials that you have. Which provided the best insulation?
Taking it further: Try different combinations of wrappings to see if these insulate the jar batter. For example, you could put some cotton between two layers of newspaper.
In designing an experiment it is always important to make it as fair as possible. In other words, apart from those things you want to study, all other conditions should remain unchanged. For example, in this experiment, the thicknesses of the wrapping materials used should be the same. Otherwise it would be impossible to say whether one wrapper kept in more heat than another because it was made of better insulating material or simply because it was thicker.
For the experiment to be fair, you must also make sure that all the wrappings completely cover the jar. Even a small opening will allow heat to escape without first having to pass through the material surrounding the glass. This kind of care is important in all experiments, since without it you bring in other factors that can have an unknown effect upon your results.
Try to think of ways to improve this experiment or extend it. For instance, in a class project, you might try to design an experiment to test the insulating properties of materials used in the building. |
Pockets of Air
 |
| Well-insulated clothing keeps us warm in
the coldest weather
|
Air is one of the best of all insulators. This means that a good insulator can be made by trapping little pockets of air within a substance. The insulation that is often found around hot-water tanks in in attics of houses works in this way. It holds still air between the fibers of a fluffy material.
You can use this idea to help stay warm in winter. By dressing in several layers of thinner clothing instead of wearing one or two thick items, you trap more air between the layers and so prevent your body heat from escaping. Boots and coats designed for use in low temperatures are themselves made from many layers for this purpose.
 |

