cyclohexane
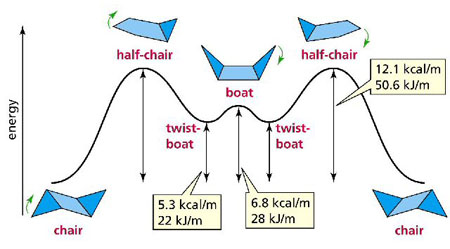
The six-member ring of cyclohexane can exist in various shapes known as conformational isomers, or conformers. The most stable conformers are the chairs; the least stable are the half-chairs. The hydrogen atoms in the molecule can assume two distinguishable positions: 'axial', when they are more or less perpendicular to the average plane of the ring; 'equatorial', when they lie close to the plane. When the chair flips from one conformation to the mirror image, axial hydrogens become equatorial and vice versa. .
Cyclohexane is a colorless liquid hydrocarbon (C6H12) that occurs naturally in petroleum (crude oil), but is made commercially by combining hydrogen with benzene, using a catalyst. Cyclohexane is a type of a compound that exist in different conformations: two forms that differ only in the arrangement of their bonds, and may "flip" from one form to the other. A sample of cyclohexane may contain both. Melting point 6°C (43°F); boiling point 81°C (178°F).
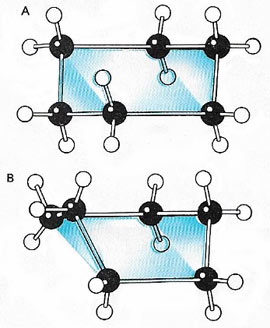 |
| The 'chair' (A) and 'boat' (B) forms of cyclohexane.
|


