dehydration
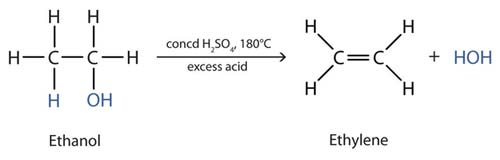
The dehydration of ethanol by hot, concentrated sulfuric acid to give ethylene (ethene).
Dehydration is the removal of water from a substance. To remove the elements of water, as in the dehydration of alcohols to ethers, requires a powerful dehydrating agent such as concentrated sulfuric acid. Generally, however, water is present as hydrate or merely absorbed, in which case milder methods suffice, such as equilibration in a desiccator with silica gel or deliquescent compounds (deliquescence). Dry air may be passed over solids in heated drums, causing evaporation. Gases are dried (for air conditioning, or before liquefying them) by compression and refrigeration. Foods are preserved by drying; this is done for convenience and compactness. Milk and eggs are dried by spraying into hot air. Modern freeze drying – sublimation of ice from frozen foods under vacuum – retains texture and flavor.
In medicine, dehydration of the body occurs through diarrhea, vomiting, cholera, or merely lack of water to replace perspiration.


