protein translation
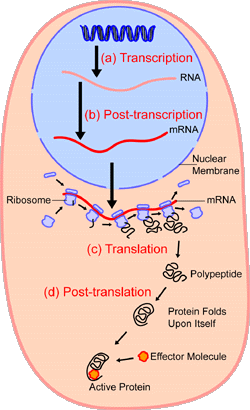
Transcription and translation.
Protein translation is the process by which proteins are made inside a cell following DNA transcription. The cellular machinery responsible for synthesizing proteins is the ribosome. A ribosome consists of structural RNA and about 80 different proteins. In its inactive state, it exists as two subunits: a large subunit and a small subunit. When the small subunit encounters a molecule of messenger RNA (mRNA), the process of translating an mRNA to a protein begins. In the large subunit, there are two sites for amino acids to bind and thus be close enough to each other to form a bond. The "A site" accepts a new molecule of transfer RNA (tRNA) – the adaptor molecule that acts as a translator between mRNA and protein – bearing an amino acid. The "P site" binds the tRNA that becomes attached to the growing chain.
The adaptor molecule that acts as a translator between mRNA and protein is a specific RNA molecule, the tRNA. Each tRNA has a specific acceptor site that binds a particular triplet of nucleotides, called a codon, and an anticodon site that binds a sequence of three unpaired nucleotides, the anticodon, which can then bind to the the codon. Each tRNA also has a specific charger protein, called an aminoacyl tRNA synthetase. This protein can only bind to that particular tRNA and attach the correct amino acid to the acceptor site.
The start signal for translation is the codon ATG, which codes for methionine. Not every protein necessarily starts with methionine, however. Often this first amino acid will be removed in later processing of the protein. A tRNA charged with methionine binds to the translation start signal. The large subunit binds to the mRNA and the small subunit, and so begins elongation, the formation of the polypeptide chain. After the first charged tRNA appears in the A site, the ribosome shifts so that the tRNA is now in the P site. New charged tRNAs, corresponding the codons of the mRNA, enter the A site, and a bond is formed between the two amino acids. The first tRNA is now released, and the ribosome shifts again so that a tRNA carrying two amino acids is now in the P site. A new charged tRNA then binds to the A site. This process of elongation continues until the ribosome reaches what is called a stop codon, a triplet of nucleotides that signals the termination of translation. When the ribosome reaches a stop codon, no aminoacyl tRNA binds to the empty A site. This is the ribosome signal to break apart into its large and small subunits, releasing the new protein and the mRNA. Yet, this isn't always the end of the story. A protein will often undergo further modification, called post-translational modification. For example, it might be cleaved by a protein-cutting enzyme, called a protease, at a specific place or have a few of its amino acids altered.


