hydrogen peroxide

Figure 1. Part of the nebular complex around Rho Ophiuchi. The region where hydrogen peroxide was found is circled. Credit: ESO.
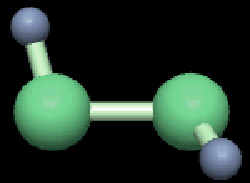
Figure 2. Ball-and-stick model of hydrogen peroxide.
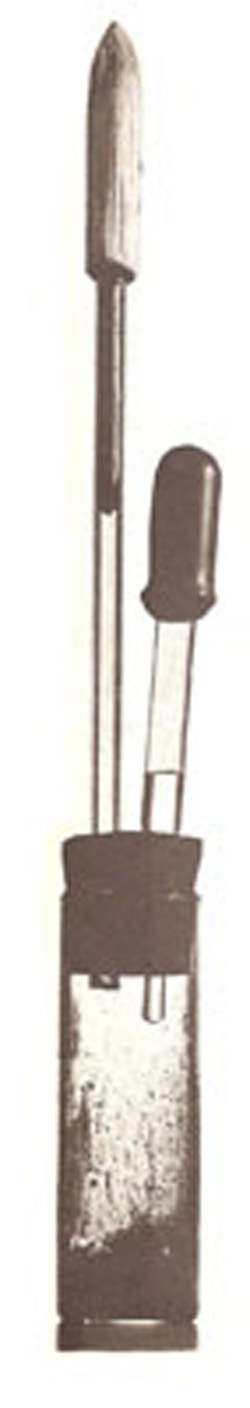
Figure 3. Hydrogen peroxide rocket apparatus.

Figure 4. Hydrogen peroxide rocket launch.
Hydrogen peroxide (H2O2) is a highly oxidizing compound of hydrogen and oxygen (Figure 2). H2O2 is a syrupy, pale blue liquid – colorless in dilute solution – and caustic to the skin. Pure hydrogen peroxide is stable, but the slightest impurity will enhance decomposition, often violently, liberating oxygen. Concentrated solutions of hydrogen peroxide are highly corrosive and toxic.
Hydrogen peroxide can act as a powerful oxidizing agent (E 0 acid solution +1.77 volts) when converted to water and as a reducing agent (E 0 +0.68 volts) when converted to oxygen (for example by potassium permanganate, KMnO4). Concentrations of H2O2 are expressed in terms of the volume of oxygen that can be liberated: 6% = 20 vol.
Hydrogen peroxide is the parent compound of peroxides. It forms adducts, perhydrates, with some salts.
H2O2 is used as a bleach, disinfectant, and deodorizer. It is prepared by electrolytic oxidation of sulfuric acid and by methods involving reduction. In the atmosphere hydrogen peroxide plays a key role in the chemistry of water and ozone, and is also probably one of the oxidizers for sulfur dioxide in cloud water droplets that produces sulfuric acid, a major component in acid rain.
| density (liquid) | 1.4 g/dm3 |
| melting point | -11°C (262.15 K) |
| boiling point | 150.2°C (423.35 K) |
| molar mass | 34.0147 g/mol |
Hydrogen peroxide in rockets
Hydrogen peroxide was also used as an oxidizer in some early liquid-propellant rocket engines, including those of the German Messerschmitt 163 (the first rocket plane ever to see flighting service) and the British Black Knight missile. Sent into turbines, steam and oxygen from hydrogen peroxide drove torpedoes, fast submarines, and the pumps which fed fuel into the firing chamber of the V-2. Explosive decomposition of peroxide in a piston-fitted launching cylinder gave the V-1 "buzz bomb" the 400 kilometers per hour (250 mph) push it needed to get underway.
Hydrogen peroxide has not been used since the late 1950s on a large scale as a propellant because it is dangerous to handle and easily decomposes, making it difficult to store. However, it has been used as a monopropellant in some small thrusters that provide attitude control on satellites.
An experiment: peroxide launches a rocket
Note: This experiment requires adult supervision.
Although 90% peroxide is restricted to research and military use, the 30% (100 volume) variety is potent enough to demonstrate the expansive force of this chemical. A little of it can be used to operate a miniature rocket launcher as illustrated. (30% hydrogen peroxide is corrosive and decomposes easily. Handle it as carefully as you would a strong acid.) 40 volume peroxide also works though not as well.
Construction is shown in Figure 3. The expansion chamber is a 2-ounce bottle closed with a 2-hole stopper. Fit a 6-inch glass tube into one of the holes and leave the other to accommodate a medicine dropper. Carve the rocket from balsa wood, and push a matchstick into it for a tail. Cover the bottom of the bottle with powdered manganese dioxide, stopper the bottle lightly (so the stopper will act as a safety valve if the gas pressure gets too high), and place the rocket tail in the tube. With the rocket out of range of anyone's eyes, insert the partly filled dropper into the open hole and squeeze out some peroxide. Instantly, the manganese dioxide breaks down the peroxide into oxygen and steam, which force the rocket upwards (Figure 4).
The rocket can be launched again and again before the bottle has to be emptied and recharged with manganese dioxide. The rocket must be a good enough fit for pressure to develop, but not too tight a fit.
Source: Science Magic by Kenneth M. Swezey, pp 6–7 (Kaye & Ward, London, 1971)
Use in medicine
Hydrogen peroxide is used, in the form of a solution or cream, as a skin disinfectant for cleansing wounds and treating superficial bacterial infections. Dilute hydrogen peroxide solution is used as a deodorant mouthwash for treating oral infections (e.g., ulcerative gingivitis). Strong solutions irritate the skin. Trade names: Crystacide, Peroxyl.
Hydrogen peroxide in space
In 2011, astronomers using the ESO-operated APEX (Atacama Pathfinder Experiment) telescope in Chile announced the discovery of hydrogen peroxide in space (Figure 1). They observed a region close to the star Rho Ophiuchi, about 400 light-years away. The region contains very cold, dense clouds of cosmic gas and dust, in which new stars are being born (see Rho Ophiuchi Nebula). The clouds are composed mostly of hydrogen, but contain traces of other chemicals, and are prime targets for astronomers hunting for molecules in space. Telescopes such as APEX, which make observations of light at millimeter- and submillimeter-wavelengths, are ideal for detecting the signals from these molecules. Using this instrument, Per Bergman, an astronomer at Onsala Space Observatory in Sweden, and colleagues, found the characteristic signature of light emitted by hydrogen peroxide, coming from part of the Rho Ophiuchi clouds.
Hydrogen peroxide is thought to form in space on the surfaces of cosmic dust grains – very fine particles similar to sand and soot – when hydrogen is added to oxygen molecules. A further reaction of the hydrogen peroxide with more hydrogen is one way to produce water. This new detection of hydrogen peroxide will therefore help astronomers better understand the formation of water in the Universe.
Hydrogen peroxide and life on Mars
It has been claimed, for example by Vance Oyama, that the presence of hydrogen peroxide on the surface of Mars would explain some of the the results of the Viking biological experiments in terms of purely inorganic chemical reactions. However, although hydrogen peroxide is known to form constantly on the surface of the moon Europa as a result of the bombardment of charged particles moving in Jupiter's powerful magnetic field, it does not appear to exist on Mars in anything like the concentration needed to account for the Viking data abiologically.
According to a very different scenario, put forward by Dirk Schulze-Makuch at Washington State University, microbes may exist near the surface of Mars that use a mixture of hydrogen peroxide and water in their cells. Purely water-based life may have originated on Mars when the planet was much wetter than it is today but as the planet cooled and dried out, the martian organisms may have adapted to their new conditions by taking in hydrogen peroxide. This would have lowered the freezing point of their intracellular solvent, enable the creatures to continue metabolizing at much lower temperatures, and would also have allowed them to harvest the small amounts of water vapor in the atmosphere, since hydrogen peroxide has a strong affinity for water. In a paper to be published in the International Journal of Astrobiology Schulze-Makuch points to an energy-capturing process similar to photosynthesis that produces hydrogen peroxide as a by-product instead of water.
The presence of microbes on Mars containing hydrogen peroxide would neatly explain the Viking biology results. For example, the oxygen and carbon dioxide that was observed in the Viking labeled release experiment would be the products of water in the LR experiment causing H2O2-rich cells to pop and, at the same time, oxidize the cells' organic material.


