iodine

Fig 1. Iodine.

Fig 2. Iodine is present in seawater in very low concentrations (0.006%). It is concentrated by seaweeds which, botanically, are green, red, and brown algae. Iodine was first discovered in 1811 when it was extracted from the ashes of seaweed. Kelp, a ribbon-like brown alga, was one of the earliest sources. Much iodine is now obtained elsewhere, for example from Chile saltpetre and brine from oil wells, although seaweed remains a valuable source of iodine.
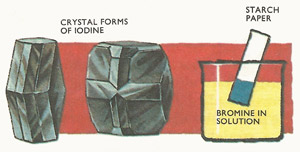
Fig 3. At normal temperatures pure iodine is a black metallic-looking solid. It can be detected in solution by the very sensitive starch test. Starch paper turns a deep blue in the presence of iodine.
Iodine (I) is a lustrous, dark gray to purplish-black, corrosive, poisonous element that is the least reactive member of the halogens (Fig 1). It readily to sublimes to a pungent violet vapor (I2 molecules). Simple solutions (e.g., in carbon tetrachloride, CCl4) are violet; solutions in which there is an appreciable charge transfer (e.g. C2H5OH, KI/H2O) are brown. Iodine reacts with other halogens (to give inter-halogen compounds), and bases (to give iodates). Chemically, iodine resembles bromine but has a greater tendency to covalency and positive oxidation states. It is large enough to form 6-coordinate oxy-anions.
Several of its isotopes are radioactive, including I-131, which is used as a tracer and in thyroid disease diagnosis and therapy. Its compounds are used as antiseptics, germicides, and in the production of dyes. Silver iodide, being light sensitive, is used in photography.
Most iodine is produced from calcium iodate (Ca[IO3]2), found in Chile saltpeter. In the US, much is recovered from oil-well brine, which contains some sodium iodide (NaI).
| atomic number | 53 |
| relative atomic mass | 126.904 |
| electron configuration | 1s22s22p63s23p64s23d104p65s24d104p5 |
| first ionization energy | 1,008 kJ/mol |
| electronegativity | 2.7 |
| atomic radius | 133 pm |
| ionic radius | 216 pm |
| melting point | 113.5°C (236.3°F) |
| boiling point | 184.4°C (363.8°F) |
| relative density | 4.93 (solid, 20 °C) |
Iodine and health
Iodine is essential for the formation of the thyroid hormones, triiodothyronine (T3) and thyroxine (T4). These hormones control the rate of metabolism and growth and development.
About 100 to 300 micrograms are needed daily. A dietary shortage of iodine may lead to goiter (enlargement of the thyroid gland) or to hypothyroidism (underactivity of the thyroid gland). Iodine deficiency in the newborn can lead to cretinism.
The amount of iodine in food depends on the amount contained in animal feed and the amount in the soil; shortages occur in limestone areas. Shortages can be overcome by consuming bread or table salt fortified with iodine or iodate.
Most plants (especially seaweeds) contain traces of iodine (Fig 2).
Medical uses of iodine
Iodine is sometimes given to people who have consumed food or drink contaminated with radioactive iodine. In such cases, absorption of the body of nonradioactive iodine reduces the absorption of the radioactive iodine.
Radioactive iodine is sometimes used to damage, and thus to reduce the activity of the thyroid gland in cases of thyrotoxicosis (a toxic condition resulting from overactivity of the thyroid gland).
Iodine compounds are used as antiseptics, in radiopaque contrast media used in some X-rays procedures and in some cough remedies.


