lanthanide

Figure 1. The lanthanides and rare earth elements.

Figure 2. 10-gram sample of cerium in an ampule under argon. Image copyright: smart-elements.com.

Figure 3. Dysprosium. Image: ESPI.

Figure 4. Erbium.

Figure 5. Europium oxide, fluorescing red under an ultraviolet light. Image copyright: smart-elements.com.

Figure 6. Gadolinium. Image copyright: smart-elements.com.

Fiureg 7. Holmium. Image copyright: smart-elements.com.

Figure 8. Lanthanum in an ampoule under argon, approx. 1cm. Image copyright: smart-elements.com.

Figure 9. Lutetium. Image copyright: smart-elements.com.

Figure 10. Neodymium. Image copyright: smart-elements.com.

Figure 11. Praseodymium. Image copyright: smart-elements.com.

Figure 12. Distilled, crystalline samarium. Image copyright: smart-elements.com.
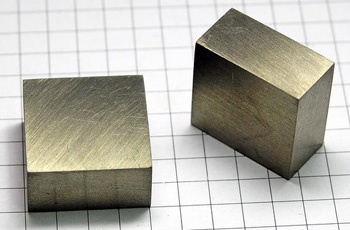
Figure 13. Terbium. Image copyright: smart-elements.com.
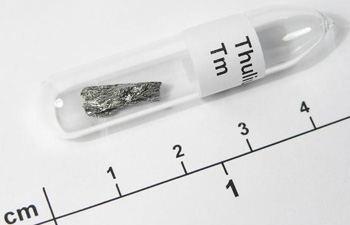
Figure 14. Thulium. Image copyright: smart-elements.com.

Figure 15. Ytterbium. Image copyright: smart-elements.com.
A lanthanide is any member of a group (called the lanthanum series) of rare, metallic elements with atomic numbers from 57 (lanthanum) to 71 (lutetium) inclusive, in which the 4f orbitals are being filled (Figure 1). The lanthanides are: lanthanum, cerium, praseodymium, neodymium, promethium, samarium, europium, gadolinium, terbium, dysprosium, holmium, erbium, thulium, ytterbium, and lutetium. Because their electronic structures are very similar, differing only in inner orbitals, the properties of the lanthanides are all very similar. The lanthanides are all reactive metals resembling scandium, forming trivalent salts and ligand complexes. Cerium, praseodymium, and terbium also form tetravalent compounds, and europium, ytterbium, and samarium form divalent ones. All form divalent ionic hydrides and sulfides.
There is a decrease (the lanthanide contraction) in ionic radii through the series, so that the third row transition element following the lanthanides have ionic radii almost identical to those of their analogues in the second row, and hence have similar properties.
The lanthanides are separated by chromatography and ion-exchange resins. They are used in alloys, including misch metal; and their compounds (mixed or separately) are used as abrasives, for making glasses and ceramics, as "getters," as catalysts in the petroleum industry, and to make phosphors, lasers, and microwave devices.
Except for promethium, the lanthanides are not uncommon – the normal source is monazite which contains 90% La, Ce, Pr, Nd in its lanthanide contents.
See also rare earth elements.
Cerium
Cerium (Ce) is a soft, ductile, iron-gray metallic element, the most abundant of the lanthanides (and more abundant than tin or lead) (Figure 2). It was first isolated by J. J. Berzelius and W. Hisinger in Vestmanland, Sweden, in 1803, and is named after the asteroid Ceres, discovered in 1801. The chief ore is monazite. Cerium is used in making pocket lighter flints), in alloys, catalysts, nuclear fuels, and special glass and ceramics, and as the core of carbon electrodes in arc lamps. However, its use is restricted by the fact that it tarnishes easily, reacts with water, and burns when heated. Its most common isotope is 140Ce (88.48%).
| atomic number | 58 |
| relative atomic mass | 140.12 |
| relative density | 6.77 |
| melting point | 798°C (1,468°F) |
| boiling point | 3,257°C (5,895°F) |
Dysprosium
Dysprosium (Dy) is a silver-white metallic element of the lanthanide series (Figure 3). Discovered by P. E. Lecoq de Boisbaudran in Paris in 1886, its name comes from the Greek dysprositos meaning "hard to get". Its chief ores are monazite and bastnaesite. Its capacity to absorb neutrons makes it important in nuclear reactor control systems; dysprosium is also in making alloys for magnets and its compounds are used in lasers. Its most common isotope is 164Dy (28.18%).
| atomic number | 66 |
| relative atomic mass | 162.5 |
| relative density | 8.54 |
| melting point | 1,409°C (2,568°F) |
| boiling point | 2,335°C (4,235°F) |
Erbium
Erbium (Er) is a silvery, metallic element of the lanthanide series, discovered by C. G. Mosander in Stockholm in 1843 (Figure 4). It is named after the Swedish town of Ytterby, where the mineral from which it was first isolated was found. There are six naturally occurring isotopes, and the chief ores are monazite and bastnaesite. Twelve radioactive isotopes have been identified. Soft and malleable, erbium is used in specialized alloys, and erbium oxide is used as a pink colorant for glass. Its most common isotope is 166Er (33.41%).
| atomic number | 68 |
| relative atomic mass | 167.26 |
| relative density | 9.045 |
| melting point | 1,522°C (2,772°F) |
| boiling point | 2,863°C (5,185°F) |
Europium
Europium (Eu) is a silver-white, metallic element of the lanthanide series, being the softest and most volatile member (Figure 5). It was first isolated as the oxide in 1896; the element itself was discovered by E. A. Demarçay in Paris in 1901 . Its chief ores are monazite and bastnaesite. Europium is used in the manufacture of color television screens, lasers, thin superconducting alloys, and control rods in nuclear reactors. Its most common isotope is 153Eu (52.18%).
| atomic number | 63 |
| relative atomic mass | 151.96 |
| relative density | 5.25 |
| melting point | 822°C (1,512°F) |
| boiling point | 1,597°C (2,907°F) |
Gadolinium
Gadolinium (Gd) is a white metallic element of the lanthanide series, first isolated as the oxide in 1880 by J. C. Galissard de Marignac in Geneva (Figure 6). It is named after the Finnish chemist J. Gadolin. Chief ores are gadolinite, monazite, and bastnaesite. A malleable and ductile metal, its specialized uses include neutron absorption (important in many nuclear reactors) and the manufacture of certain alloys for making magnets and in the recording heads of video recorders. Its most common isotope is 158Gd (24.87%).
| atomic number | 64 |
| relative atomic mass | 157.25 |
| relative density | 7.898 |
| melting point | 1,311°C (2,392°F) |
| boiling point | 3,233°C (5,851°F) |
Holmium
Holmium (Ho) is a metallic element of the lanthanide series (Figure 7), first identified spectroscopically in 1878 by P. T. Cleve in Uppsala, Sweden, and independently by M. Delafontaine and J. L. Soret in Geneva. Its chief ore is monazite (a phosphate). Alloys of holmium are in used in magnets but the element has few commercial uses. Its most common isotope is 165Ho (100%).
| atomic number | 67 |
| relative atomic mass | 164.93 |
| relative density | 8.803 |
| melting point | 1,470°C (2,680°F) |
| boiling point | 2,720°C (4,930°F) |
Lanthanum
Lanthanum (La) is a silver-white metallic element that is the prototype and second most abundant member of the lanthanide series (Figure 8). First identified by C. G. Mosander in Stockholm in 1839, its name comes from the Latin lanthana meaning 'to lie hidden.' Its chief ores are monazite and bastnaesite. Soft, malleable, and ductile, lanthanum is used as a catalyst in cracking crude oil, in alloys, and to manufacture optical glasses. Its most common isotope is 139La (99.91%).
| atomic number | 57 |
| relative atomic mass | 138.906 |
| relative density | 6.17 |
| melting point | 920°C (1,688°F) |
| boiling point | 3,454°C (6,249°F) |
Lutetium
Lutetium (Lu) is a metallic element of the lanthanide series, first isolated by G. Urbain in Paris in 1906 (and independently by C. James in New Hampshire), together with ytterbium (Figure 9). Its name comes from Lutetia, the Latin word for Paris. Its chief ore is monazite. The element is used as a catalyst, but has no other commercial uses. Its most common isotope is 175Lu (97.41%).
| atomic number | 71 |
| relative atomic mass | 174.97 |
| relative density | 9.842 |
| melting point | 1,656°C (3,013°F) |
| boiling point | 3,315°C (5,999°F) |
Neodymium
Neodymium (Nd) is a silver-yellow metallic element of the lanthanide series (Figure 10). It was first isolated in the form of its oxide in 1885 and the pure metal was first obtained in 1925. Chief ores are monazite and bastnaesite. Neodymium is used to manufacture lasers, and its salts are used to color glass. Its most common isotope is 142Nd (27.11%).
| atomic number | 60 |
| relative atomic mass | 144.24 |
| relative density | 7.004 |
| melting point | 1,010°C (1,850°F) |
| boiling point | 3,068°C (5,554°F) |
Praseodymium
Praseodymium (Pr) is a silver-yellow metallic element of the lanthanide series, first isolated in 1885 (Figure 11). Its chief ores are monazite and bastnaesite. Soft, malleable, and ductile praseodymium is used in carbon electrodes for arc lamps. Its most common isotope is 141Pr (100%).
| atomic number | 59 |
| relative atomic mass | 140.908 |
| relative density | 6.77 |
| melting point | 931°C (1,708°F) |
| boiling point | 3,512°C (6,354°F) |
Promethium
Promethium (Pm) is a radioactive, metallic element of the lanthanide series. It was first made in 1941 by particle bombardment of neodymium and praseodymium. Promethium occurs in uranium ores in minute amounts. The isotope 147Pm is used in phosphorescent paints, X-ray tubes, and in nuclear-powered batteries for space vehicles. The most stable isotope is 145Pm (half-life 17.7 years).
| atomic number | 61 |
| melting point | 1,080°C (1,976°F) |
| boiling point | 2,460°C (4,460°F) |
Samarium
Samarium (Sm) is a gray-white metallic element of the lanthanide series, first identified spectroscopically in 1879 (Figure 12). Its chief ores are monazite and bastnaesite. Samarium is used in carbon-arc lamps, as a moderator in nuclear reactors, and as a catalyst. Some samarium alloys are used for making powerful permanent magnets. Samarium's most common isotope is 152Sm (26.72%).
| atomic number | 62 |
| relative atomic mass | 150.35 |
| relative density | 7.52 |
| melting point | 1,072°C (1,962°F) |
| boiling point | 3,791°C (3,256°F) |
Terbium
Terbium (Tb) is a silver-gray metallic element of the lanthanide series (Figure 13). Discovered in 1843, it is found in such minerals as monazite, gadolinite, and apatite. The soft element is used in semiconductors; sodium terbium borate is used in lasers. Its single natural isotope is 159Tb .
| atomic number | 65 |
| relative atomic mass | 158.925 |
| relative density | 8.234 |
| melting point | 1,360°C (2,480°F) |
| boiling point | 3,041°C (5,506°F) |
Thulium
Thulium (Tm) is a lustrous, silver-white, metallic element and the least abundant member of the lanthanide series (Figure 14). First discovered in 1879, its chief ore is monazite. Soft, malleable, and ductile, thulium combines with oxygen and the halogens. It is used in arc lighting; thulium-170, which emits X-rays, is used in portable X-ray units. Its most common isotope is 169Tm (100%).
| atomic number | 69 |
| relative atomic mass | 168.934 |
| relative density | 9.31 |
| melting point | 1,545°C (2,813°F) |
| boiling point | 1,947°C (3,537°F) |
Ytterbium
Ytterbium (Yb) is a silver-white, metallic element of the lanthanide series (Figure 15). First isolated in 1878, ytterbium's chief ore is monazite. The shiny, soft element is used to produce steel and other alloys. Its most common isotope is 174Yb (31.84%).
In 2009,a team of scientists announced it had managed to teleport a single ion of ytterbium – a world's first. Previously, teleportation had been restricted to photons. The group conducted teleportation between two ions of ytterbium (Yb+): an original that held a unique quantum state, and a second 'blank' target ion.1
| atomic number | 70 |
| relative atomic mass | 173.04 |
| relative density | 6.97 |
| melting point | 824°C (1,515°F) |
| boiling point | 1,193°C (2,179°F) |
Reference
1. Olmschenk, S. et al. Science 323, 486–489 (2009).


