radioactive series
A radioactive series is a series of radioactive nuclides in which each member of the series is formed by the decay of the nuclide before it. The series ends with a stable nuclide. Four radioactive series are known. Three of these occur naturally; the other one starts with an artificial radionuclide.
Introduction
The element uranium is the heaviest atom found in nature, and is the only element with all its natural isotopes radioactive. Since an isotope differs from other isotopes of the same element in having a different relative atomic mass (r.a.m.), or atomic weight, it may be defined by the name of the element to which it belongs and the r.a.m. of the isotope. Thus uranium-238, which is the most common uranium isotope, has 92 protons (the atomic number of uranium is 92) and 146 neutrons in each atomic nucleus and therefore has a r.a.m. of 238. Uranium-235, the next most common uranium isotope, has 3 neutrons less in each nucleus.
These are the two most common uranium isotopes. They are both unstable and therefore decay radioactively into other elements by emitting charged particles from the nuclei of their atoms. Instead of decaying into stable atoms, they decay into atoms which are themselves radioactive. These then decay into a different atom and the process is repeated until a stable atom is reached. A system where atoms decay through a series of elements in this way is called a radioactive series.
Natural radioactive series
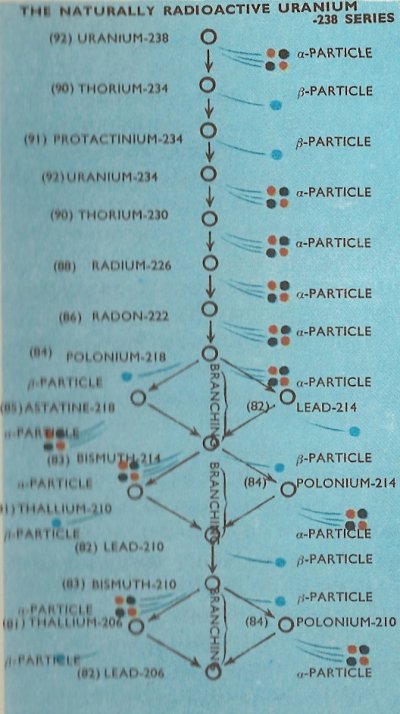
There are three entirely separate radioactive series found in nature, the longest of which begins with the decay of uranium-238 and is known as the naturally radioactive uranium-238 series.
The second naturally occurring radioactive series originates with the second
most commonly occurring isotope of uranium, uranium-235, and this is called
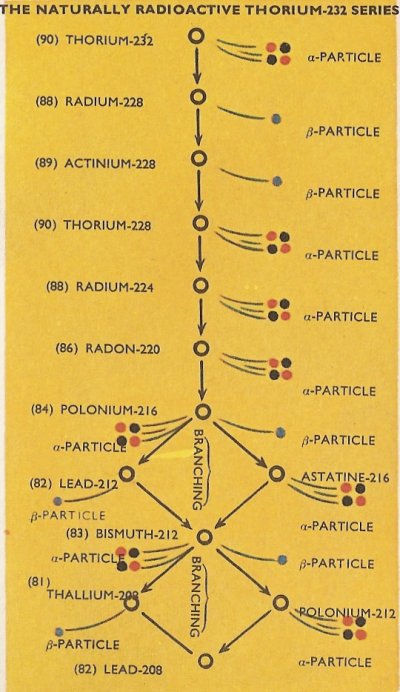
the uranium-235 series. The third series is the naturally radioactive thorium-232 series.
These series arise because of the loss of either a beta
particle or an alpha particle from
an atom, a process which changes the charge on the nucleus of the decaying
atom. When a beta particle is lost, it means that the charge on the nucleus
(and therefore the atomic number of the atom) is increased by one. When
an alpha particle is emitted the atomic number of the atom is reduced by
two and its atomic weight by four.
The newly-created atom then emits a particle to become an atom of a different element which then itself decays into yet another different atom.
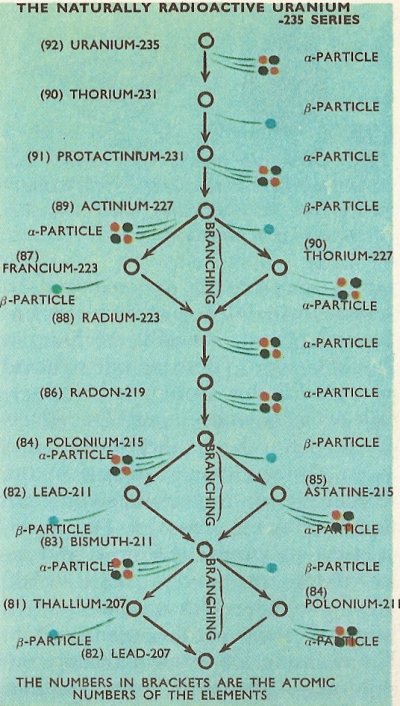
Most often, all atoms of a particular radioactive isotope of an element emit either alpha particles and no beta particles, or all beta particles and no alpha particles. Some members of the radioactive series have, however, some atoms which emit alpha particles and some which emit beta particles. (No single atom emit both alpha and beta particles.) In these cases, a branch occurs in the radioactive series, with some of the decaying atoms converted into another element. For example, in the uranium-235 series, actinium-227 decays either into francium-223 by loss of an alpha particle, or into thorium-227 by loss of a beta particle. Both of these then decay, the francium by loss of a beta particle, and the thorium by loss of an alpha particle, into radium-223. The radium-223 produced by either route is identical, and the branch in the series is thus closed. The radium then decays to produce the next member in the series, radon-219.
The complete details of the three naturally occurring series are as in the diagrams, with the branchings which occur in the series shown.
It will be noticed that all of the natural series terminate with isotopes of lead, i.e., lead-206, lead-207, and lead-208. It happens that all of these are stable isotopes of the element and no further radioactive emission therefore takes place.
The times taken (as measured by the half-life) for the various radionuclides in the radioactive series to decay differs widely. For example the time taken for half an amount of uranium-238 to decay to thorium-234, which is the first step in the uranium-238 series, is 4.5 × 109 (4,500 million) years. By contrast, the half-life of thorium-234 is only 24.5 days, and the half-life of the next member in the series, protactinium-234, a mere 1.14 minutes.
An artificial series
After the discovery of the three radioactive series of naturally found isotopes, many physicists searched in the hope of finding more series. In 1940 new elements were artificially made which had atomic numbers greater than 92, the atomic number of uranium. These were called transuranic elements. The transuranic elements neptunium (atomic number 93) and plutonium (atomic number 94) were the first to be produced and isolated. In 1945 others were discovered, including americium (atomic number 95). These three elements are radioactive in any of their isotopic forms, and since they are produced artificially, they are said to be artificially radioactive.
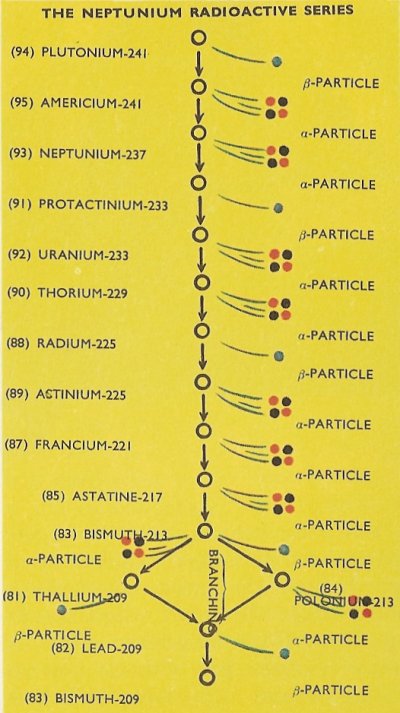
Later it was realized that a fourth radioactive series does exist – the neptunium radioactive series – but it cannot be called a naturally radioactive series since it contains some transuranic elements.
The neptunium radioactive series (so-called because neptunium-237 is the most stable radioactive isotope in the series) is shown in the bottom diagram. As with the naturally radioactive series, alpha particle and beta particle emission causes the decay of the radioactive isotopes. Branching again occurs with a bismuth isotope, in this case where some atoms of bismuth-213 decay by alpha particle emission to thallium-209 atoms, while others decay by beta particle emission to atoms of polonium-213. Thallium-209, by beta particle emission, and polonium-213 by alpha particle emission, both decay to identical atoms of lead-209, which decays to bismuth-209, a stable isotope, which forms the end of the neptunium radioactive series.
As with the naturally radioactive series, a quantity of any isotope in the series will eventually decay to become a similar quantity of the stable isotope – in this case bismuth-209.
Most of the natural radioactive isotopes take their places in a radioactive series. Only seven of the naturally found radioactive isotopes do not appear in one of the three naturally radioactive series. Forty-six isotopes do appear in these three series – all of which are isotopes of the elements with atomic numbers between 81 and 92. In contrast, most of the artificial radioactive isotopes do not belong to a radioactive series.


