FROM GLASSES TO GASES: The Science of Matter - 1. Matter of Interest
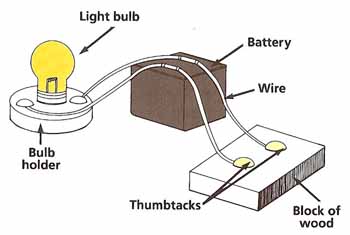
Figure 1. Conductivity test.
Figure 2. Experimenting with floating and sinking.
Think about how many different materials we use in our everyday lives –
wood, paper, plastic, metal, glass, cotton, rubber, stone, and dozens of
others. Some of these materials are natural, the rest are human-made.
 |
| Matter is all around us,
in both human-made and natural things
|
How we use a substance depends upon its properties. For instance, rubber works well for the sole of an athletic shoe because it bends and then goes back to its original shape. It also grips well and is very tough. Glass, on the other hand, would make a terrible shoe, but is ideal for use in windows.
Scientists spend a lot of time trying to develop new materials to do new jobs or to do old jobs better. This has led to such breakthroughs as nonstick frying pans, toughened glass, heat-resistant tiles for the space shuttle, and even clothes that change color.
Three States of Matter
Everything around us – every material or substance – is made of MATTER. Matter is anything that takes up space. It can exist in three states: SOLID, LIQUID, or GAS. Under normal conditions, most substances occur in just one of these states. For example, we think of iron as being a solid. But if it is heated enough, iron will turn into a liquid, and eventually into a gas. As a substance changes its state, so do many of its properties.
All substances are made up of tiny particles called ATOMS. In most substances, atoms are joined together in groups known as MOLECULES.
Molecules are always moving. In a solid, the molecules are very close together and can only vibrate in fixed positions. In a liquid, the molecules are usually a bit farther apart and can move around one another. In a gas, the molecules are far apart and are able to dart rapidly in any direction.

Sorting MaterialsYou will need:
What to do:
Choose one of the materials (see Figure 1). What is its color? Is it rough, dull, or shiny? Use the nail to find out whether the material is easy to scratch. Does the material float when placed in the bowl of water? Is it attracted by the magnet?
Connect the light bulb to the battery. Join two of the wires to the battery and then to the block of wood, using the thumbtacks as shown. Test to see if electricity will pass easily through the material by laying it across the thumbtacks. The bulb will light up if electricity can get through.
Repeat these tests with the other materials. Make a table of your results under the headings shown. Arrange the materials in groups according to their properties. For example, you might group the materials under the headings "Easy to Scratch" and "Hard to Scratch" and the under the headings "Will Float" and "Will Not Float." Do the materials that scratch easily also tend to be the ones that will float? If so, can you suggest an explanation? Make other such comparisons and try to explain your results.
Taking it further: Think about other properties that materials possess. Devise your own experiments to test these properties.
Collect several pieces of different kinds of fabric. Design an experiment to find out which of the fabrics is the hardest-wearing.
It is interesting to look at the ideas that different people come up with when asked to design an experiment. Take the case of an experiment to measure which group of fabrics is the most hard-wearing. Different pupils in a class will design different ways to carry out this test. Which is the best? Sometimes an idea for an experiment does not work well in practice because the equipment is too complicated, or is simply wrong for the job. In other cases, the experiment does not isolate the topic of interest properly. If other factors are allowed to interfere with the results in an unpredictable way, the experiment is not fair."
One method of finding out how well various fabrics wear is to use
a brick and a thick newspaper (to help keep the brick in place). Put
the newspaper on a table and place the brick in the center of the
newspaper. Hold each piece of fabric at either end and rub it backward
and forward across the brick. Keep a record of how many rubs it takes
to make a small hole in the material. Your test will only be fair
if the rubs are equally hard and you use pieces of fabric the same
size. Does a cotton sweater last longer than a woolen one? Does brand
A of jeans wear better than brand B? Set goals for your experiment
and present your results in a clearly written report. |
Little and Large
Atoms and molecules are much too small to be seen individually by the human
eye. But we can certainly see the effect of trillions and trillions of these
tiny particles together.
The properties of a substances are related to the properties of its molecules.
For example, a hard-wearing material is one whose molecules are bound firmly
together. A material, such as steel, that can be turned into a magnet is
one whose molecules can behave like miniature magnets. A substance that
is yellow contains molecules that reflect mostly yellow light.
Diamond: A Hard Act to Follow
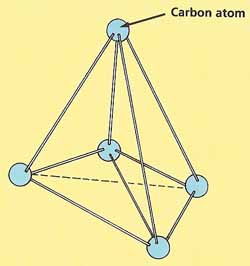 |
| In diamond, carbon atoms are arranged in
a the shape of pyramids
|
Diamonds, pencil lead, and charcoal may not look much alike, but they are all made from the same kind of atoms: carbon atoms, In diamonds, the carbon atoms are linked by very strong bonds. This makes diamonds extremely hard. Another reason for their hardness is that the atoms are not arranged in layers, so they cannot slide over one another. Each carbon atom is at the center of a triangular pyramid, surrounded by four other atoms at the corners of the pyramid. This pattern repeats itself millions and millions of times, so a diamond is really a single giant molecule.
Diamonds are the hardest substance known. This makes them ideal for use in cutting equipment, such as glass cutters and diamond-edged saws. Powdered diamonds are also used as abrasives for smoothing very hard materials. Diamonds are found in rocks in certain parts of the earth, but they can also be made artificially in laboratories. Good, natural diamonds are extremely rare and are used mostly in jewelry.

Floating IdeasYou will need:
What to do:
Weigh each object in your hand. How heavy does it feel? Put it in the bowl of water. Does it float or sink? How well does it float, or how quickly does it sink? Keep a record of your results. Investigate other materials in the same way See Figure 2). |
Dense, Denser, Densest
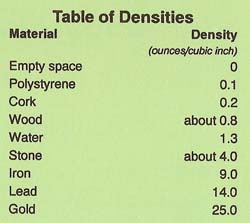 |
Polystyrene and cork feel light. Metals feel heavy. This is because metals are more dense.
Density is a measure of how much matter is contained within a certain volume. Water has a density of 1.3 ounces per cubic inch. Anything will float in water if its density is less than the density of water. Look at the table of densities and you will see why a cork floats and a stone sinks.
Fruits and vegetables contain mostly water, so they either sink or float. Fro example, apples and oranges ten to float, but carrots and tomatoes tend to sink. Oranges sometime sink when peeled because the fruit is denser than the peel.
A substance may be dense because it contains heavy atoms, or because its atoms are packed closely together, or for both of these reasons. Are denser substances harder than less dense ones? Some they are. For example, stone is harder than polystyrene. On the other hand, lead and gold are both much softer than iron, even though they have higher densities. Hardness is decided not just by how well a substance's atoms are packed together but by how strongly they are bonded.
 |

