FROM GLASSES TO GASES: The Science of Matter - 4. How Slow the Flow?

Figure 1. Water has a low viscosity.
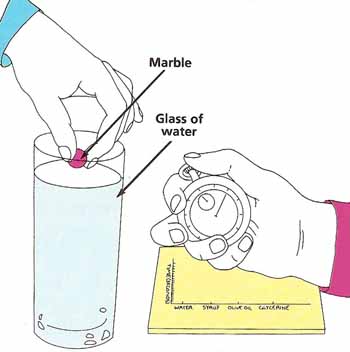
Figure 2. An experiment with viscosity.
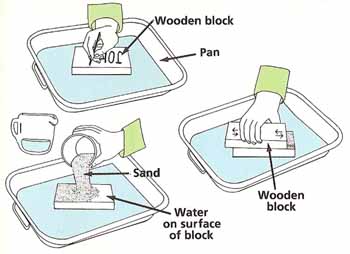
Figure 3. Rate of wear experiment.
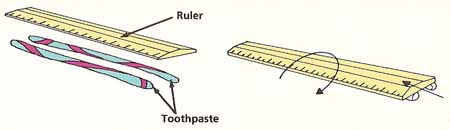
Figure 4. Toothpaste experiment experiment.
Water is very different from honey, syrup, glycerine, or oil. It pours easily and is not thick and sticky like the others. The property that determines how easily a liquid pours is called VISCOSITY. Water has a low viscosity; syrup has a high viscosity. Liquids with a high viscosity are said to be viscous.
"Slower than Molasses in January"
You may have been called that if you've ever dawdled too long over a job. Molasses looks like dark syrup – and it flows just about as slowly. When cold, it flows especially slowly. So "molasses in January" is a good way to let people know that should get a move on.
All liquids are more viscous when cold than when hot. This is because as a liquid heats up, its molecules move about faster, so they are able to slide past one another more easily.

The Long DropYou will need:
What to do: Fill the jar with water. Hold the marble so that it is just touching the surface. Let go and immediately start the stopwatch. Record how long it takes for the marble to reach the bottom (see Figure 2). Repeat this with each of the other liquids, remembering to clean the marble and jar thoroughly before each test. Draw a bar graph chart showing your results, with the bar showing the shortest time on the left and that showing the longest on the right. Which is the most viscous substances?
Warm the syrup in a pan until its temperature is about 100° (38°C). Pour the syrup in the jar and time how long it takes the marble to reach the bottom. What can you deduce from your results? |
Engines and Oil Cans
A car engine has many fast-moving and close-fitting metal parts. If these parts were to rub directly against one another, they would quickly overheat and wear out. This is why all engines need oil. The oil – a smooth, viscous liquid – acts as a cushion between the moving pieces of metal. It also carries heat away from especially hot parts and spreads it more evenly throughout the engine.

Rate of WearYou will need:
What to do:
Using the marker pen, write your name in large letters across one of the pieces of wood. Lay the wood on the tray. Pour some water across the wood and then sprinkle some sand on top. Dip one face of another piece of wood in water and then lay this face across the first block. Rub the top piece of wood 100 times backward and forward against the bottom piece while pressing down steadily. Wipe off the bottom piece with the rag and set it aside (see Figure 3).
Write your name, exactly as before, on another piece of wood and place it in the tray. Pour a thin layer of olive oil across the wood and then sprinkle the same amount of sand on as your used before. Apply another thin layer of olive oil to the last piece of wood. Repeat the rest of the test as before.
Compare the two pieces of wood that you had marked with your name. Which has been worn the most? Can you explain your findings? |
Sticky and Springy
Some substances, including toothpaste, sit in solid lumps until they are given a push. Then they flow like a liquid. Such substances are called plastic liquids.
Toothpaste is both sticky and springy. Liquids that have these two properties are said to be viscoelastic. So toothpaste is both plastic and viscoelastic.
Most kinds of toothpaste also have tiny, hard particles inside them. These particles help scrape away the sugary layer that clings to teeth, without damaging the teeth themselves. When tiny solid particles hang in a liquid, the result is called a SUSPENSION. Toothpaste is a suspension of solids in a plastic, viscoelastic liquid.

Strange PasteYou will need:
What to do:
Hold the tube of toothpaste upside down. Squeeze gently until the toothpaste sticks out about ¼ inch below the nozzle (see Figure4). Stop squeezing. What happens?
Put a small blob of toothpaste on your finger. Tip the blob sideways, then upside down. Rub the toothpaste between your fingers. Do you think the toothpaste behaves more like a solid or a liquid?
Squeeze out two lines of toothpaste, 4 inches long and ¾ inch apart on a smooth, clean surface. Lay the ruler along them. Push the end of the ruler gently, first along the direction of the lines and then across the lines. What happens when you let go each time? Invent a theory about how toothpaste molecules behave that would explain all your observations. |
Jelly in the Can
Some years ago, special new paints, called thixotropic paints, were developed. They look like jelly in a can. You can drip a brush into them, lift the brush out, and the paint does not drip. But as soon as you move the brush against a hard surface, the paint comes off like a slippery liquid. A thixotropic liquid is like a gel until you disturb it. Then it flows freely. This kind of paint has a warning label "Do Not Stir," because if you stir, you break the thixotrope, and the paint runs and drips.
Ketchup is also thixotropic. That is why it often gets stuck in the bottle. By shaking the bottle you break the thixotrope and allow the sauce to run out.

