FROM GLASSES TO GASES: The Science of Matter - 3. Liquids with Skins

Figure 1. Pond skater.
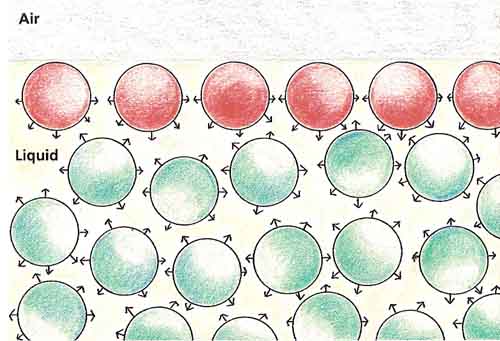
Figure 2. Molecules at a liquid's surface.
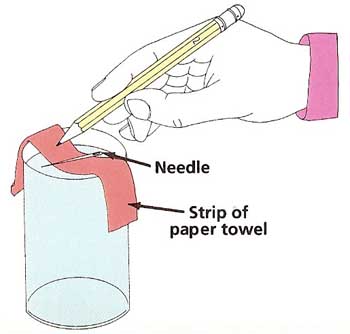
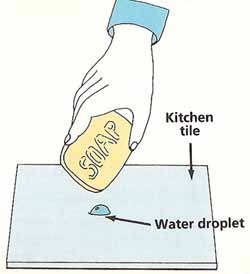
Figure 3. Testing the strength of surface tension.
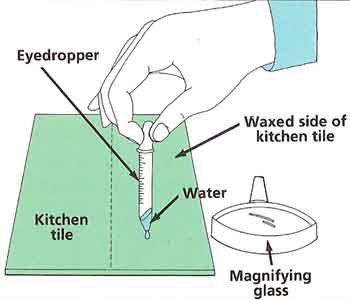
Figure 4. Testing the strength of surface tension.
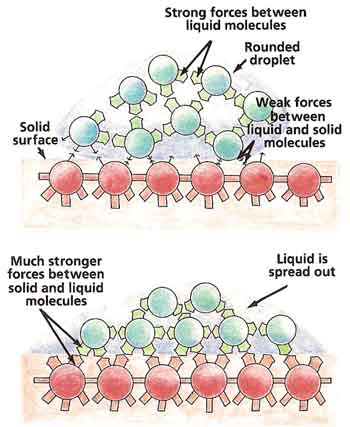
Figure 5. The stronger the force between a liquid and a solid surface, the more the liquid spreads out.
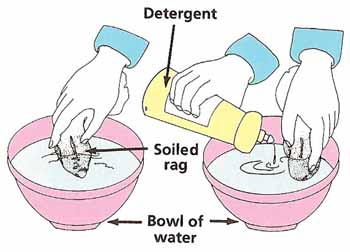
Figure 6. 'Breaking the tension' experiment.
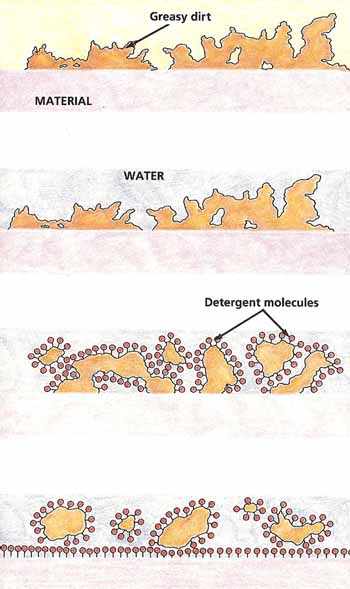
Figure 7. Soap molecules have two "ends"; one end is attracted by water and the other is repelled by it.
Visit a pond in the summer and you are likely to see pond skaters dashing about over the surface (see Figure 1). These little creatures depend for their lives on being able to stand up on and run across water. Even though the pond skater is very light, you might expect that its feet would sink into the water. But water behaves as if it has a tight elastic skin. The pond skater's legs press a little way into this skin, but don't break through. Instead the skin bends for them.
The property of liquids that makes them seem to have a skin is known as SURFACE TENSION.
What causes surface tension?
The molecules of a liquid are always trying to pull one another together. In the middle of a liquid, a molecule is pulled equally hard from all directions, so the attractive forces cancel out. But a molecules on the surface is only pulled downward. This downward tug draws the surface of the liquid tightly together, so it appears to have a skin (see Figure 2).
Water molecules attract one another quite strongly, so water has a fairly strong skin. That is, it has a high surface tension. The molecules of some other liquids, such as alcohol, pull less hard on one another, so the liquid's skin is weaker.

How Strong Is a Liquid's SkinYou will need:
What to do: Almost fill the glass with water. Make sure the needle is perfectly dry and free of grease. Use the strip of paper towel as a sling to the lay the needle on the water. Do this very carefully and gently. Use the pencil to poke down the paper (see Figure 3). Does the needle float? If not, dry it off and try a few more times. Repeat the experiment with the paperclip and the nail. Do they float or sink?
Replace the water with alcohol. Again, try to float the needle, the paperclip, and the nail. What are your results? Can you explain them?
Taking it further:
Repeat this experiment using other liquids, such as milk or a solution
of salt or sugar in water. Which has the strongest skin? |

Water DropletsYou will need:
What to do:
Wash the kitchen tile and dry it off thoroughly. Put some wax polish on the rag and rub the wax onto just one half of the tile. Fill the eyedropper with water. Carefully put one drop onto the unwaxed half of the tile and one drop onto the waxed half. Look at both drops from the side with the magnifying glass (see Figure 4). Sketch what you see. Can you explain your observations?
Taking it further:
Try putting a drop of water onto a number of other different waterproof surfaces. Only use materials that are clean and will not soak up the water. On which surfaces does the water spread out the most and on which does it form the roundest drops? Repeat the experiment using hot water and other liquids.
After you have read the section "Molecular Tug-of-War," you may understand why water spreads out more on some surfaces than on others. Water molecules pull one another together by a force called COHESION. They may be attracted to the molecules on a sold surface by a force known as ADHESION. Raindrops on a window pane stick together by cohesion and to the glass by adhesion.
What happens if the adhesive force between a liquid and a solid is very small? The liquid pulls itself into droplets. Most furniture polish contains silicones. Silicones come in various forms – oils, greases, resins, and substitutes for rubber. They are all human-made, they all have a "backbone" of silicon atoms, and they all have the property that they will not attract water. Therefore, water on silicone wax forms into round beads and water on a silicone-coated garment runs off without wetting the fabric.
Water and glass molecules, on the other hand, stock together quite well. Pour some water into a drinking glass. Can you see how the water at the edge climbs a little way up the sides? This because of the adhesion of the water and the glass. Try putting a thin glass tube into a container of water. What happens to the water? The effect you see is called CAPILLARY ACTION (a thin glass tube is known as a capillary tube). Capillary action is what allows water to travel up the stems of plants. You can see it even more clearly using two small squares of glass. Put a strip of card between the pieces of glass at one end to space them apart and a rubber band around them to hold them together. Wet the glass squares and then dip them into a dish of water colored with food coloring. What happens? Try to understand what you see in terms of the adhesive force between glass and water. |
Molecular Tug-of-War
Liquid molecules are not only attracted to one another, but they may also be attracted to the molecules of solid surfaces with which they come into contact. This explains why raindrops stick to windows (see Figure 5).
If the liquid molecules are pulled more strongly by the solid surface than they are by one another, the liquid will tend to spread out. On the other hand, if the liquid molecules are drawn more strongly to one another than to the solid surface, the liquid will gather itself into small, round droplets. Water forms droplets on wax because the pull between one water molecule and another is much greater than the pull between a water molecule and a wax molecule.
The lower the surface tension of a liquid, the more it tends to spread out over a solid surface. Also, the surface tension of a liquid usually decreases as the temperature increases.

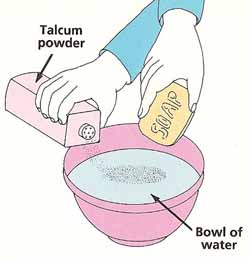
Breaking the tensionYou will need:
What to do:
Using the eyedropper, gently squeeze a drop of water onto the kitchen tile. Look at the shape of the drop from the side. Touch the bar of soap to the water drop. What happens?
Partly fill the bowl with water. Lightly sprinkle the talcum powder onto the surface so that it is evenly spread. Touch the bar of soap to the middle of the bowl and watch what happens. Can you explain your observations?
Taking it further
Empty the bowl and refill it with water. Take one of the clean rags and soil it with a few drops of cooking oil and dirt from the ground. Put the dirty rag into the bowl and swirl it around for a minute. Spread out the rag to dry.
Take the other rag and rub in roughly the same amount of oil and dirt as before. Replace the water in the bowl with fresh water and a small amount of detergent. Swirl the rag around for a minute and then set it out to dry. When both rags are dry, compare them (see Figure 6). Which is the cleaner? Can you explain what has happened? You might want to take this experiment further by comparing the effects of different kinds of detergents on various types of stains. Does hot water help to clean better than cold water? If so, why? |
Coming Clean
After a long summer day, you probably feel like taking a shower. But water on its own does not do a very good job of removing the grease and dirt that stick to your skin. To get clean, you need to rub on soap as you wash. But how does soap work?
Soap weakens the surface tension of water. This lets the water spread out and actually touch the surface it is contact with.
On its own, water would not be enough, because the natural oils on your skin, which trap dirt, will not dissolve in water. Fortunately, soap molecules have a special structure. One end of them is attracted by water, the other is repelled by it. What happens is that the water-repelled ends of soap molecules stick to your skin and to any greasy dirt that is on it. The water-attracted ends are turned toward the water. The result is that the soap molecules lift and surround the grease so that it can be easily washed away (see Figure 7).
The detergents used for washing dishes work in the same way. They have been developed by scientists to have even better lathering and cleaning properties than ordinary soap.
 |