adsorption
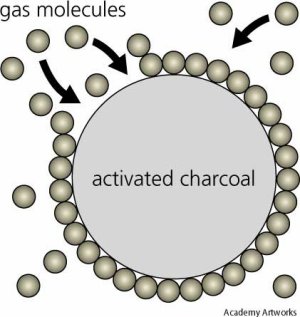
Adsorption on the surface of activated charcoal.
Adsorption is a process in which molecules of gas, of dissolved substances in liquids, or of liquids adhere in an extremely thin layer to surfaces of solid bodies with which they are in contact. The degree of absorption depends on temperature, pressure, and the surface area. The forces binding the absorbate to the absorbent (solid surface) may be physical or chemical; chemical adsorption is specific, and is used to separate mixtures (see chromatography). Adsorption is used in gas masks and to purify and decolorize liquids.
Adsorption, which involves a usually weak and reversible binding, is different than absorption, in which the pores of a solid are filled. The commonest industrial adsorbents are activated charcoal, silica gel, and alumina (aluminum oxide), because they have extremely large surface areas per unit mass.


