bromine
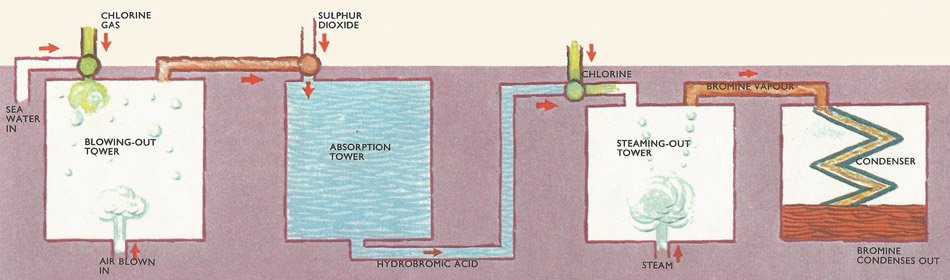
Extraction of bromine from brine liquid. Chlorine is passed into the brine and replaces chlorine from the bromide salts. The bromine is blown out with an air blast and the bromine-laden air is mixed with sulfur dioxide. Hydrogen bromide is formed and this is mixed with chlorine which again releases bromine. This is steamed-out in the steaming out tower and passes on to the condenser.

Bromine.
Bromine (Br) is a heavy, volatile, corrosive, reddish-brown, nonmetallic liquid element (see nonmetal), having a pungent and highly irritating vapor. Bromine is one of the halogens, and has properties intermediate between those of chlorine and iodine. It occurs as bromides, mainly in seawater, from which it is extracted by oxidation with chlorine.
Industrial preparation of bromine
To obtain bromine industrially from brine liquid, the salt is extracted from the seawater and the remaining liquor passed down a reaction tower, while chlorine gas flows up it in the opposite direction. The chlorine reacts with the magnesium bromide in the liquor:
MgBr2 + Cl2 → MgCl2 + Br2
This reaction provides an example of the greater activity of the lighter members of the halogen family. Although the magnesium and bromine atoms are fairly tightly bound in magnesium bromide, the greater electronegativity of the chlorine atoms is sufficient to make them oust the bromine atoms from the compound.
Uses of bromine and bromides
Soluble metal bromides (see halides) are used as sedatives; silver bromide, being light-sensitive, is used in photography. Ethylene dibromide, the chief bromine product, is used as a lead scavenger in antiknock additives. Alkyl bromides (see alkyl halides) are used as fumigants and solvents.
| atomic number | 35 |
| relative atomic mass | 79.904 |
| electron configuration | 1s22s22p63s23p64s23d104p5 |
| first ionization energy | 1,140 kJ/mol |
| electronegativity | 3.0 |
| atomic radius | 114 pm |
| melting point | -7.2°C (19.0°F) |
| boiling point | 58.78°C (137.8°F) |
| relative density | 3.119 |


