uranium

A billet of highly enriched uranium recovered from scrap processed at the Y-12 Facility in Oak Ridge, TN. Credit: US Dept of Energy.
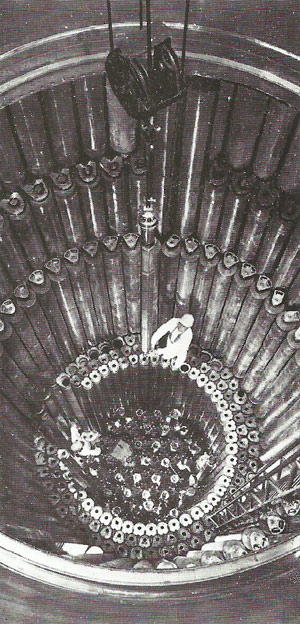
Reactor pile containing uranium fuel rods.
Uranium (U) is a heavy, soft, silvery-white, metallic element, radioactive, easily oxidized, and having 14 known isotopes of which 238U is the most abundant in nature. It has the highest atomic number (92) of any naturally occurring metal. Uranium is in the actinide series. It occurs in several ores, including pitchblende (uraninite), carnotite, autunite, and torbernite from which it is extracted and processed for use in research and nuclear fuels. Uranium was discovered in 1789 by Martin Kaproth.
The naturally occurring isotopes are fissionable 235U (0.7205% of natural uranium), 238U which cannot be fissioned with thermal neutrons (99.2739% of natural uranium), and 234U, a decay product of 238U (0.0056%). Enriched uranium is that in which the percentage of the fissionable isotope 235U has increased beyond the content of 0.7205% of natural uranium. Depleted uranium has a lower percentage of 235U than that occurring in natural uranium; it is produced during uranium isotope separation.
The decay of uranium yields a series (see radioactive series) of radioactive products, including radium and radon. Exposure to the radiation emitted by uranium can cause tissue damage or cancer. Uranium is also chemically poisonous and can cause damage to the urinary system.
| atomic number | 92 |
| relative atomic mass | 238.03 |
| melting point | 1,132°C (2,070°F) |
| boiling point | 3,818°C (6,904°F) |
| relative density | 18.95 |
 |
| Uranium is found in many ores, including carnotite shown here. |
Uranium oxide
One of a series of compounds of which UO2, U4O9, U3O8, and UO3 are the most common, and of which U3O8 is the most stable. The latter is green, brown, or black with an orthorhombic crystalline structure.


