atomic theory of matter
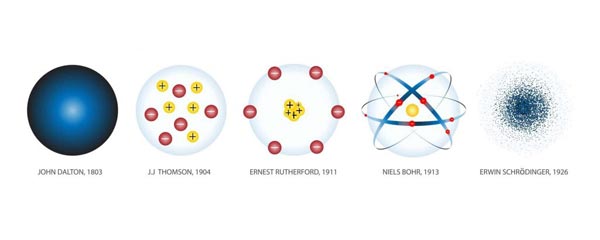
Certain ancient Greek philosophers were the first to suggest that all matter might consist, at a very small level, of indivisible, indestructible particles. But this theory of atomism was purely a philosophical movement, entirely separate from the much more modern scientific theory of atoms. It was the English chemist John Dalton, in 1803, who first demonstrated on a scientific basis that matter is atomic in nature. Ten years later, the English chemist and physician William Prout (1785–1850) suggested that the different kinds of atoms are built up out of the simplest, hydrogen.
The discovery of the periodic table of the elements by Dmitri Mendeleyev pointed to the existence of some recurrent structure factor within the atoms themselves, hinting at the probable existence of subatomic components. In the hands J. J. Thomson, the study of the discharge of electricity through rarefied gases led to the discovery of the electron, which were subsequently found to be constituents of every kind of atom. Electrons carry a charge which is the smallest that can be observed in nature, all charges being simple multiples of the elementary unit, the electronic charge.
Soon after the discovery of the electron, research by Ernest Rutherford in the new science of radioactivity led him to conclude that an atom is formed out of a massive small nucleus around which rotate the relatively light electrons. Later research revealed that the individual atomic nuclei are made up of different arrangements of protons and neutrons, which are, themselves, in turns out, comprised of even smaller particles called quarks.
The knowledge that matter is atomic prepared the way for the development of Max Planck's quantum theory, which demonstrated that radiation too has a particle-like aspect. According to the quantum theory, as elaborated by Einstein, electromagnetic radiation travels in small discrete packets, called quanta.


