hydrogen bond
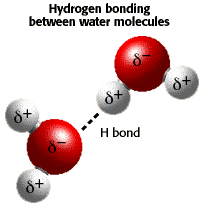
A hydrogen bond is a weak chemical force that can operate both within and between certain types of molecule. A hydrogen bond results when a hydrogen atom that is attached to another atom by a covalent bond is also attracted to a neighboring electronegative atom (such as oxygen or nitrogen), either in the same molecule or in a separate molecule.
Although only about 10% as strong as covalent bonds, hydrogen bonds are crucial to life as we know it. They are responsible for many of the unique properties of water, for the tertiary structure of proteins, and for the interaction between purines and pyrimidines which contributes to the stability of the DNA double helix. Hydrogen bonding is also important in the hydrogen halides, ice, alcohols, oxy-acids, ammonia, amines, and amides.


