insulin
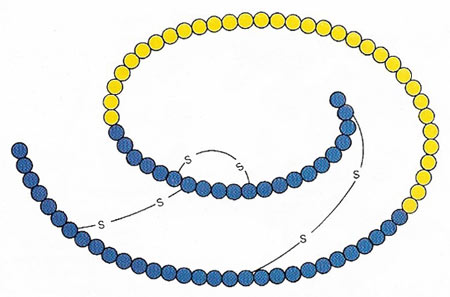
Figure 1. Insulin is formed by the removal of 33 amino acid uits (yellow) from the protein proinsulin. The two chains of the insulin molecule are bound covalently by sulfur-sulfur bridges.
Figure 2. Large insulin crystals grown in space. Credit: NASA.
Insulin is a protein hormone, secreted by the beta (or B) cells of the islets of Langerhans in the pancreas, which promotes the uptake of glucose by body cells, particularly in the liver and muscles, and thereby controls its concentration in the blood. Insulin is the only hormone which reduces the level of sugar in the blood and is secreted in response to a rise in blood sugar (e.g., after meals, or in conditions of stress); the sugar is converted into glycogen in the cells of muscle and the liver under the influence of insulin.
Insulin is a tiny protein consisting of two linked polypeptide chains derived from a single proinsulin chain. The two chains, "A" consisting of 21 amino acids and "B" of 30 amino acids, are linked by two disulfide bridges (Figure 1). Insulin was the first protein to have its amino acid sequence determined (in 1955).
Underproduction of insulin results in the accumulation of large amounts of glucose in the blood and its subsequent excretion in the urine. This condition, known as diabetes mellitus, can be treated successfully by insulin injection. If insufficient insulin is taken, diabetic coma may result, while in excess hypoglycemia supervenes; both require prompt medical treatment.
The isolation of insulin as a pancreatic extract by F. G. Banting and C. H. Best in 1921 was a milestone in medical and scientific history. Insulin in medicine used to be extracted from animals. Today, it can be produced artificially by inserting the genes of human insulin into bacteria which multiply in fermentation plants.


