thermal conduction
Thermal conduction is the transmission of heat from places of higher temperature to places of lower temperature in a substance, by the interaction of atoms, molecules, or ions, or electrons possessing greater kinetic energy with those possessing less. In gases the heat energy is transmitted by collision of the gaseous molecules, those possessing the greater kinetic energy imparting, on collision, some of their energy to molecules having less. Conduction in liquids is mainly due to the same process. In solid electrical conductors, the chief contribution to thermal conduction arises from a similar process taking place between the free electrons present. The interaction of the molecules responsible for thermal conduction in solid electrical insulators arises from the elastic binding forces between the molecules, which are effectively fixed in space.
The rate of heat transfer by conduction is determined by the temperature difference (ΔT ), length (l ), cross-sectional area (A ), and thermal conductivity of the material (k) according to the following formula:
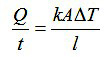
Conductivities are usually given in units of W/m K. They are highest in metallic solids, lower in nonmetallic solids, very low in liquids, and extremely low in gases. The best ordinary metallic conductors are silver (429 W/m K), copper (401), gold (317), aluminum (237), and tungsten (174). Among nonmetallic solids, diamond is best (895); most silicate minerals have low values (< 2) The material with the greatest thermal conductivity (105!) is the superfluid helium II, which only exists at temperatures below 2.17 K.
Thermal conductor
A thermal conductor is a material that conducts heat well and quickly, i.e., has a relatively high thermal conductivity. Most metals are good thermal conductors but the best thermal conductors of all are diamond and carbon nanotubes. The reason for this is that both diamond and carbon nanotubes contains strong molecular bonds in very regular order making it easy for molecular vibrations to travel quickly and efficiently through the materials.
Thermal insulation
Thermal insulation is the reduction of the transfer of heat from a hotter area to a colder one. Thermal insulation is used for three distinct purposes: to keep something hot; to keep something cold; and to maintain something at a roughly steady temperature. Heat is transferred in three ways, conduction, convection, and radiation. The Dewar flask, or vacuum bottle, thus uses three different techniques to reduce heat transfer: a vacuum between the walls to combat conduction and convection; silvered walls to minimize the transmission of radiant heat from one wall and maximize its reflection from the other; and supports for the inner bottle made of cork, a poor thermal conductor.
A thermal insulator is a material that does not conduct heat well, i.e., has a relatively low thermal conductivity. Plastics, wood, cork, and some fabrics are good thermal insulators. The reason is that they contain weak molecular bonds in disorderly arrangements. Heat is transfered in a material by the vibration of the atoms and molecules. A disorderly arrangement of particles and bonds slows down the passage of heat through the material.


