copper

Figure 1. Native copper. Credit: David Belanger, UCSC.

Figure 2. Antlerite from Chuquicamata, Chile.

Fiureg 3. Azurite. Image source: Mineralogical Information Institute.

Figure 4. Bornite. Image source: Mineralogical Information Institute.

Figure 5. Chalcanthite.

Figure 6. Chalcocite. Image source: Mineralogical Information Institute.

Figure 7. Chalcopyrite. Image source: Mineralogical Information Institute.

Figure 8. Covellite.

Figure 9. Cuprite. Image source: Mineralogical Information Institute.

Figure 10. Malachite. Image source: Mineralogical Information Institute.

Figure 11. Alloys of copper find many uses in marine engineering. High-tensile brass, as that of ships' propellers, is based on Muntz metal containing about 40% of zinc. Other elements are often added to promote particular properties for various uses. To produce the range of high-tensile brasses, tin, aluminum, iron, and manganese may be among the additives used; products are sometimes known as manganese bronzes although technically they are brasses.

Figure 12. Cupronickel coins.

Figure 13. Verdigris.
Copper (Cu) is a ductile, malleable, reddish-brown metallic element in group 11 (old group IB) of the periodic table; it is a transition element. Copper is of great importance because of its high electrical conductivity (only silver, among pure metals, conducts electricity better at room temperature) and thermal conductivity. It was probably the first metal ever to be extracted from its ores.
Copper was named from the Greek word kyprios, that is, the Island of Cyprus, where copper deposits were mined by the ancients. The chemical symbol for copper is Cu which is derived from the Latin name for copper, cuprium.
| atomic number | 29 |
| relative atomic mass | 63.546 |
| electron configuration | 1s22s22p63s23p64s13d10 |
| main oxidation states | +1, +2 |
| relative density | 8.96 |
| melting point | 1,084°C (1,983°F) |
| boiling point | 2,567°C (4,653°F) |
Occurrence
Copper is mainly found in the form of compounds but, as might be expected of a fairly unreactive metal, it is sometimes found as the element itself (called native copper, Figure 1). There are more than 140 naturally occurring copper compounds but only a few of them (chalcopyrite, chalcocite, cuprite, antlerite, azurite, bornite, and malachite) are important as ores from which copper is extracted. Most of them occur only in small quantities. Many are compounds of sulfur because copper and sulfur were originally thrown up together in volcanic regions.
Antlerite
Antlerite (Figure 2) is a green mineral consisting of basic copper sulfate (Cu3[OH]4SO4). A minor copper ore of widespread occurrence, it is formed by oxidation of primary copper minerals. It is named for the Antler mine, Arizona, where the first specimens were collected.
Antlerite crystallizes in the orthorhombic system in crystals up to 2 centimeters long. They commonly appear fibrous and in cross-fiber veinlets, feltlike, granular or powdery lumps and aggregates.
It is found in association with brochantite, atacamite, chalcanthite, kröhnkite, natrochalcite, linarite, and gypsum. Deposits occur in Arizona, New Mexico, California, Nevada, and Alaska, USA; Chihuahua, Mexico; various locations in Chile; near Zwickau, Saxony, Germany; Caldbeck Fells, Cumbria, and at several mines in Cornwall, England; and Lerida, Spain.
Azurite
Azurite (Figure 3) is a basic copper carbonate mineral found in the oxidized parts of copper ore veins, often as earthy material with malachite. The crystals are blue, brilliant, and transparent. In the past the gemstones were used as pigments in wall paintings. Hardness 3.5–4, specific gravity 3.77–3.89.
Bornite
Bornite (Figure 4) is an opaque, reddish-brown sulfide mineral of iron and copper (Cu5FeS4). It is also called "peacock ore" because of its tarnish when fractured. Bornite is found as masses and sometimes as crystals (cubic system) in intrusive igneous rocks and metamorphic rocks. It occurs widely, with concentrations in Chile, Peru, and Tasmania. It alters to chalcocite. Hardness 3, relative density 5.0.
Chalcanthite
Chalcanthite (Figure 5) is a mineral that consists of mainly of hydrated copper sulfate (CuSO4), although it is rarely used as a source of copper. It occurs as greenish-blue triclinic crystals or as fibrous veins or stalactites. It is soluble in water and has a nauseating taste. Hardness 2.5; relative density 2.25.
 |
| Chalcanthite belongs to the triclinic system. The unit cell has three axes of different lengths, none of which is at right-angles. |
Chalcocite
Chalcocite (Figure 6) is a dark gray or black mineral, copper (I) sulfide (Cu2S). It is a major copper ore found mainly in sulfur deposits. The crystals occur in orthorhombic granular masses, or rarely in prismatic form. It is found (often with bornite) in the Ural Mountains, Africa, South America, Alaska, Connecticut, and the southern United States. Hardness 2.5 to 3.0; relative density 5.7.
Chalcopyrite
Chalcopyrite (Figure 7), also called copper pyrites, is the most important copper ore (CuFeS2), very similar to pyrite, with which it is often associated in sulfide veins in igneous and certain metamorphic rocks. The crystals are tetragonal but often occur in masses. Hardness: 3.5–4; relative density 4.2. Chalcopyrite is found in the United States, Canada, Australia, western Europe, and South America.
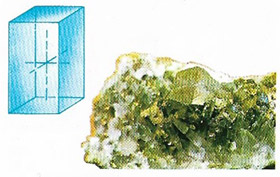 |
| Chalcopyrite belongs to the tetragonal crystal system. The unit cell is a straight prism on a square base. |
Covellite
Covellite (Figure 8) is a mineral form of copper sulfide (CuS). It occurs with other copper minerals and is mined as an ore. It forms hexagonal, platy crystals, which have a deep indigo blue color, often tinged with purple. It cleaves into thin, flexible plates. Hardness 1.5–2.0, relative density 4.7.
Cuprite
Cuprite (Figure 9) is a reddish-brown, brittle, translucent mineral consisting of copper (I) oxide (Cu2O); a major ore of copper. Its octahedral, dodecahedral, and cubic crystals occur in the cubic system, and it also occurs in grains. It is formed by oxidation of copper sulfide ores and so is commonly found near the surface. Cuprite is found in Europe, Australia, Arizona, southwest Africa, and South America. Hardness 3.5–4; relative density 6.1.
Malachite
Malachite (Figure 10) is a green mineral consisting of basic copper (II) carbonate (Cu2CO3[OH]2). It occurs widely, usually with azurite, and is formed by weathering of other copper minerals. A minor ore of copper, it is used for ornamental stone and gems.
Extraction
Most copper is extracted from copper pyrites, also known as chalcopyrite. The raw copper extracted from ores is not pure enough for electrical purposes and is further purified by electrolysis. A block of impure copper is immersed in copper sulfate solution and connected to the positive terminal of a direct current supply (i.e., as the anode) and a thin sheet of pure copper is connected to the negative terminal as the cathode. Copper ions drift across the solution from the anode to the cathode and while the anode is eaten away the cathode grows as pure copper is deposited on it. The impurities either go into solution or drop down to the bottom. The gold and silver in the mud which collects underneath the anode is often sufficient to pay for the refining process. Gold and silver are closely related to copper and tiny quantities are often found with it.
Alloys
Copper is made into a great many alloys of which bronze (copper and tin) is probably the best known; it is much harder wearing than pure copper. The coming of bronze changed the entire way of life of Paleolithic (stone age) man. The new, sharp bronze weapons made the killing of animals for food much easier and made organized warfare possible. A number of copper alloys are called bronzes, though they need not contain tin: copper + tin + phosphorus is phosphor bronze, and copper + aluminum is aluminum bronze. Other important alloys of which copper is a major component are brass, germ an silver, cupronickel, and beryllium copper (Figure 11).
Cupronickel
Cupronickel is an alloy containing 75 parts of copper to 25 parts of nickel, widely used for coins (Figure 12). In 1947 it replaced the silver alloy used until that time for non-copper coins in the UK. Cupronickel is fairly strong and yet resistant to corrosion and abrasion and at the same time readily worked into shape.
Chemistry
Copper has three complete shells of electrons and only one electron in its outer shell. From this it would be expected to have a valence (combining power) of one, and in fact copper does have a valence of one in a series of compounds known as copper (I), or cuprous, compounds. Each copper (I) ion has lost its outer electron, making the ion positively charged. Strangely enough copper (I) ions are very unstable, for although copper should have a valence of one, it prefers to have a valence of two, An electron from an inner shell is lost as well as the outer one making a copper (II) or cupric, ion. Copper (I) compounds are very unstable and easily become copper (II) compounds, which are much more stable.
Copper compounds give a greenish-blue coloration to the flame of a Bunsen burner (see Figure 14)and most of the salts are blue or greenish-blue in color. Copper (II) sulfate (CuSO4.5H2O), or blue vitriol, is a blue crystalline solid occurring naturally as chalcanthite. Copper compounds are also poisonous. For this reason copper sulfate solution is used for killing unwanted fungi growing on vines.
 |
| Figure 14. Copper flame test. |
Copper (II) ions have a tendency to gather round them four molecules of water, both when in solution and when in solid crystalline form. This water is loosely bound and if crystals of copper sulfate, for example, are heated the water is driven off and the crystal structure collapses. Ammonia also tends to group itself around copper (II) ions. Copper (II) salts will dissolve in ammonia solution to form a complex cuprammonium compound in which four molecules of ammonia are grouped around each copper (II) ion.
Copper oxide
Copper oxide exists in two forms: copper(I) (cuprous) oxide (Cu2O), a brilliant red powder found in nature as the mineral cuprite; and copper(II) (cupric) oxide (CuO), which is black and decomposes into copper(I) oxide and oxygen when heated. It is added to furnace melts as an oxidant.
Copper sulfate
Copper sulfate also exists in two forms. Copper(I) (cuprous) sulfate (Cu2SO4) is a light gray powder that reacts instantly with atmospheric moisture to produce copper(II) (cupric) sulfate. This is usually seen as the bright blue crystals of the pentahydrate, blue vitriol (CuSO4.5H2O), used for copper plating, for preserving wood, and for killing algae in ponds. It can also be dehydrated to the colorless anhydrous salt (CuSO4), which, because it absorbs water and turns blue, is used as a desiccant and, more widely, as a moisture indicator.
Verdigris
Verdigris is a blue-green powder, which consists of basic copper (II) acetate (Fig 13). It is made by pickling copper in acetic acid and is used as a pigment and a mordant in dyeing. The term is also applied to the blue-green coating, or patina, that exposed copper acquires in air over a period of time. It is usually a basic copper carbonate, but near the sea will be a basic copper chloride.
Copper and life
Copper is an essential nutrient to all higher plants and animals. In animals, it is found primarily in the bloodstream, as a cofactor in various enzymes, and in copper-based pigments. The copper-containing pigment hemocyanin performs the oxygen-carrying task in the blood of various mollusks and arthropods that hemoglobin does in our own bodies.
Copper poisoning is rare, occurring mainly in people who drink home-made alcohol distilled using copper tubing. Symptoms of poisoning include nausea, vomiting, and diarrhea. Copper excess may also result from Wilson's disease, a rare inherited disorder of copper metabolism.
Copper use in prehistory
Early humans were probably attracted by the bright appearance of native copper and probably discovered that it could be hammered into various shapes. The earliest artifacts of metal are Egyptian pins and beads made of beaten copper. In time it was realized that copper can be cast into any shape when it is molten and can be given a good edge. The earliest fused implements are from c. 4000 BC in Egypt and Babylonia. Implements of copper are not common in Europe because the metal is scarce there and existing objects were melted down when bronze was introduced. The discovery of copper smelting helped contribute to human progress since it release people from dependence on rare native copper and allowed them to make more tools by using the relatively plentiful copper ores. It is likely that about 2,000 years separated the initial use of hammered copper and copper smelting and casting.
The use of native copper marks the start of each of the metal cultures, since silver and gold, though malleable, are not industrially useful to early man. Once it was discovered that the soft copper could be hammer-hardened, its use began to spread. When it was observed that too much hammering made the copper brittle, heat was probably used to remedy this problem.
Source: Winick, Charles. Dictionary of Anthropology. Totowa, New Jersey: Littlefield, Adams & Co. (1970).


