diffusion
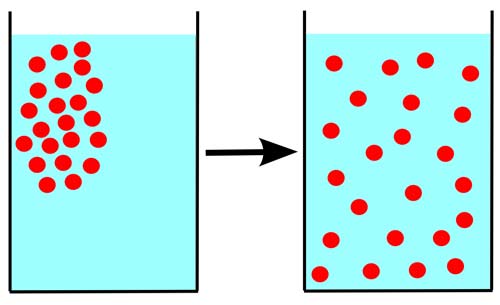
Some particles are dissolved in a glass of water. At first, the particles are all near one top corner of the glass. If the particles randomly move around ("diffuse") in the water, they eventually become distributed randomly and uniformly from an area of high concentration to an area of low, and organized.
Diffusion is the spontaneous mixing of one substance with another when in contact or separated by a permeable membrane. Diffusion is a result of the random motions of their component atoms, molecules, ions, or other particles. Diffusion occurs most readily in gases, less so in liquids, and least in solids. Diffusion rates increase with increasing temperature.
Thomas Graham showed that the rate of diffusion of different gases through a porous diaphragm is inversely proportional to the square roots of their densities (or molecular weights). This law, called Graham's law of diffusion, is the basis of a method of separation of gases, and has been applied successfully to the separation of hydrogen and deuterium.
A diffusion mechanism is also used in dialysis as a means of separating colloids from crystalloids. The rate of diffusion of molecules in gels is practically the same as in water, indicating the continuous nature of the aqueous phase. The diffusion of gases into a stream of vapor is of importance in diffusion pumps.
Thermal diffusion
Also known as the Soret effect, thermal diffusion is diffusion caused by a temperature difference setting up a concentration gradient in a fluid.


