ethane
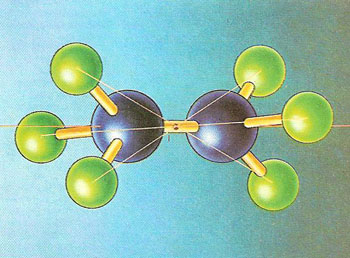
A ball-and-stick model of the ethane molecule, made up of two carbon atoms (blue spheres) and six hydrogen atoms (green spheres). In terms of symmetry, it has a center of inversion (I) but the two methyl (CH3) groups are not related by a mirror plane between them. These groups can mutually rotate into alignment, giving the molecule such a mirror plane but destroying the center of inversion. (Molecular symmetry and its alteration with internal motion dominates much of chemical theory,
Ethane (C2H6) is the second member of the alkane series of hydrocarbons. Ethane occurs in natural gas and is one of the products of petroleum cracking. It is a colorless, odorless, flammable gas, which forms an explosive mixture with air.
Ethane may be prepared by reduction of ethene or ethyne by hydrogen under pressure in the presence of a nickel catalyst, or by the electrolysis of a solution of potassium ethanoate.
Ethane is used in low-temperature refrigeration plant. It has been detected as a trace component in the atmospheres of Jupiter, Saturn, Uranus, and Neptune, and in the atmosphere of Saturn's largest moon Titan.
| molecular weight | 30.1 |
| melting point | -182.7°C (90.3 K) |
| boiling point | -88.6°C (184.5 K) |
Ethyl compounds
Ethyl compounds are organic compounds containing the ethyl group, C2H5. The most important ethyl compounds are ethanol, diethyl ether, ethyl acetate, lead tetraethyl, and the ethyl halides (see alkyl halide).


