galactose
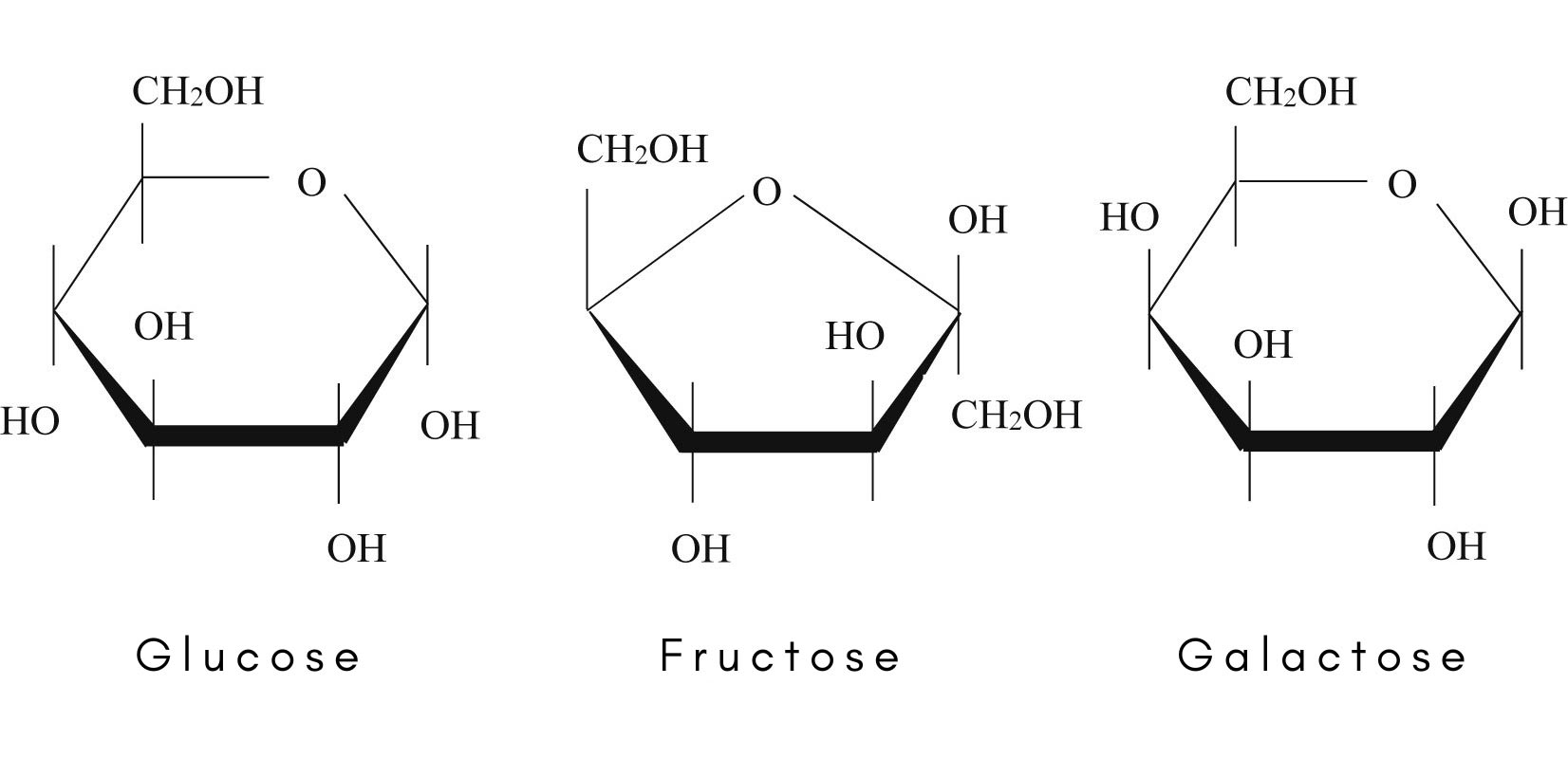
Comparison of the molecular structures of glucose, fructose, and galactose. Galactose and glucose are stereoisomers. Galactose, glucose, and fructose are structural isomers.
Galactose (C6H12O6) is a monosaccharide or simple sugar, stereoisomeric (see stereoisomers) with glucose, that is essential in the diet and found in abundance in the diet, especially in dairy products, where it coexists with the milk sugar lactose. People who are lactose-intolerant may also be lacking in galactose, however it can easily be obtained from other foods, including many fruits and vegetables. Before it can be utilized by the body for energy, it must be converted into glucose by the liver. The enzyme necessary for this conversion is missing in infants with a rare inherited metabolic disease called galactosemia.
Lactose is also a building block of carbohydrate chains associated with glycoproteins and glycolipids. In the human body, glucose is changed into galactose in order to enable the mammary glands to secrete lactose.
Galactosemia is an inborn inability to utilize galactose, which in consequence accumulates in the blood. Untreated, affected infants fail to thrive and show developmental delay, but if galactose is eliminated from the diet growth and development may be normal.


