lactic acid
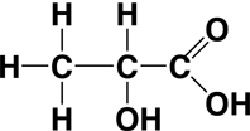
Lactic acid is an organic acid, (2-hydroxypropanoic acid, CH3CH(OH)COOH), formed as a product of glycolysis – the break down of glucose by anaerobic metabolism (chemical processes that don't require oxygen). Glycolysis is an essential step in the utilization of the energy in food by many animal cells, and particularly during contraction of skeletal muscle (also known as striped or striated muscle) in vertebrates. Lactic acid is also formed as a result of the metabolism of many bacteria, for example, from lactose in the souring of milk (hence its name).
Muscle tissue which is being vigorously exercised can't obtain sufficient oxygen from the blood to supply the energy it needs. Under this condition of anaerobic exercise additional energy can be obtained by the reduction of glucose to lactate. This lactate tends to build up in the muscle and is responsible for the cramps which are experienced in vigorous exercise. Lactic acid is also produced in tissues when they receive insufficient oxygen due to impairment of their blood supply in heart attack (myocardial infarction) or shock.
Normally, lactic acid is removed from the blood by the liver. If lactic acid accumulates, a condition called lactic acidosis results.
Lactic acid is a colorless, crystalline solid (relative density 1.206; melting point 18°C; boiling point 122°C) or syrupy liquid, that occurs in three stereoisomeric forms (see stereoisomers. dl-lactic acid, a mixture of equal amounts of (dextrorotatory) d-acid and (levorotatory) l-acid, is formed by the action of certain bacteria on lactose, as mentioned above. The d-form, sarcolactic acid, occurs in muscle tissue. The optically inactive dl-form is used in dyeing and tanning.


