allotropy
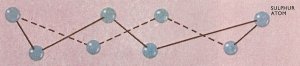
Figure 1. Crystals of both rhombic and monoclinic sulfur are built up from zigzag rings of eight atoms as shown. However, these units are arranged in different ways in the two allotropes.
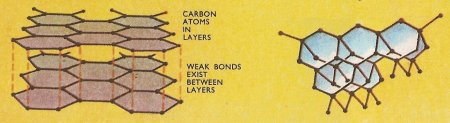
Figure 2. The two common allotropic forms of carbon: graphite (left) and diamond.
Allotropy is the existence of an element in two or more forms, known as allotropes, in the same state (solid, liquid, or gas). The physical properties (color, crystalline form if solid, density, etc.) may differ widely, but identical chemical compounds can be formed from the various allotropes of the one element. Allotropy in which the various forms are stable under different conditions and are reversibly interconvertible at certain temperatures and pressures, is called enantiotropy.
Notable examples of allotropy include diamond and graphite, oxygen and ozone, sulfur, and phosphorus.
Enantiotropic allotropy
In the solid state sulfur can exist in two different crystalline allotropes – rhombic and monoclinic (Figure 1). At temperatures below 95.5°C the rhombic form is stable, and any needle-shaped crystals of monoclinic sulfur which cool below this temperature will be gradually converted into the rhombic form. Conversely, the monoclinic form is stable between 95.5°C and the melting point (119.25°C), and when maintained at a temperature in this range the rhombic form slowly changes into monoclinic. However, if rhombic sulfur is heated rapidly it will melt at 112.8°C to yield an amber-colored liquid. Rhombic and monoclinic sulfur are said to be enantiotropic allotropes, since one form is stable below a certain definite temperature or transition point while the second form is stable above this temperature. As the names of these two allotropes imply, the principal difference between them is in their crystalline structures.
Monotropic allotropy
In contrast, graphite is the only stable solid allotrope of carbon, and at all temperatures diamond is very, very slow in changing into graphite (Figure 2). Any such pair of allotropes is said to be monotropic if only one form is stable over the whole range of temperatures. It may, therefore, seem strange that diamond shows a greater resistance to chemical attack and has a stronger molecular structure.
All the differences between the two allotropes may be attributed to the distinct arrangements of the carbon atoms in space. In diamonds each carbon atom is linked by valence bonds of equal length to four adjacent carbon atoms, so that the diamond lattice extends in all three dimensions. Graphite has a plate-like structure – each carbon atom is linked to three more carbon atoms in the one plane while the fourth much longer bond forms a weak link with an atom in the next plane. Thus the valence bonds in graphite do not hold the carbon atoms together so tightly as they do in diamond – compare the result of drawing a piece of graphite (pencil "lead") and diamond across a sheet of paper. The hard diamond bites into the paper whereas the graphic crystals shear off in layers. The structure accounts also for the higher electrical conductivity of graphite. Likewise graphite, although comparatively unreactive, takes part in chemical reactions more readily than diamond. If heated sufficiently in air or oxygen, both allotropes yield carbon dioxide and there is no difference between the molecules of this gas formed from the two allotropes.
For another example of monotropic allotropy, see phosphorus.
Dynamic allotropy
There is a third type of allotropy, known as dynamic allotropy. In this, the two forms exist in equilibrium. The proportion of the two allotropes that are in equilibrium with one another varies with temperature and sometimes with pressure. The two liquid forms of sulfur exhibit this type of allotropy, and the change from one form to the other is accompanied by a change in color and its viscosity (resistance to flow).
As sulfur is heated above its melting point, the amber mobile liquid becomes darker in color and much more viscous (thick). The viscosity reaches a maximum at about 180°C. Further heating produces a much darker, almost black, liquid which gradually becomes less viscous.
The two liquid allotropes are called λ-sulfur and μ-sulfur. At temperatures close to the melting point the proportion of λ-sulfur is quite high, but as the temperature rises more and more of the λ-form changes into μ-sulfur. The molecules of both forms contain eight atoms arranged in a ring, which ruptures on heating to yield chains still containing eight atoms. The increase in viscosity at 180°C is attributed to their interaction between these chains.
Atoms of oxygen can exist in molecules containing two or three atoms. the former (O2) is also called oxygen while the latter (O3) is ozone. These molecules also exhibit dynamic allotropy, though it appears that the equilibrium is heavily weighted to the O2 molecule at normal atmospheric pressure, i.e. there are many more oxygen than ozone molecules in the mixture. An increase in pressure favors the formation of ozone, since this causes a reduction in the number of molecules to be accommodated: 3O2 → 2O3. Explosive substances are formed when (contrary to instructions) grease is applied to the valves on cylinders of compressed oxygen. It seems likely that the explosive compounds are ozonides, the ozone having been formed as a result of the oxygen being compressed.


