anodizing
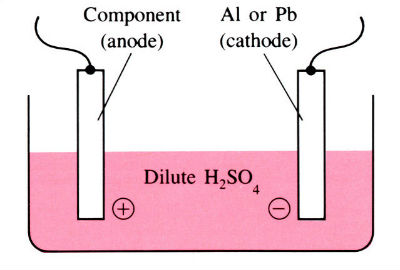
Aluminum and its alloys have some inherent resistance to atmospheric corrosion due to the presence of a thin protective oxide film 2.5–10 nm thick. Anodizing is an electrolytic process for producing much thicker oxide coatings, 5–30 μm thick, with improved physical and chemical properties. The components are made the anode in a dilute acid solution. The oxygen liberated at the anode face results in the formation of a coherent oxide film which is very adherent to the base metal.
Anodizing is a process for building up a corrosion-resistant or decorative oxide layer on the surface of a metal (usually aluminum or magnesium) object. The item to be anodized is made the anode in a cell containing an aqueous solution of sulfuric, chromic, or oxalic acid as electrolyte. The desired oxide coating is formed when a current is passed through the cell (see electrolysis). Further treatment can render this oxide layer waterproof, electrically insulating, or brightly colored.


