electrolysis
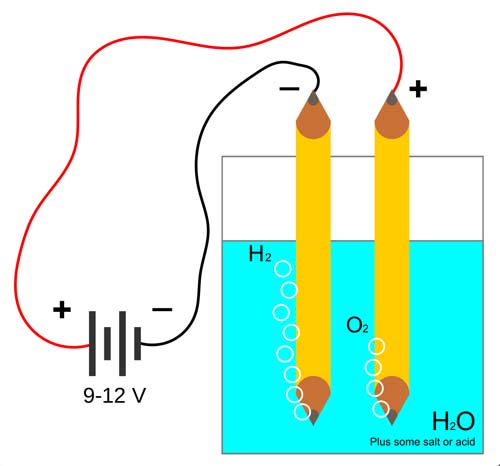
A simple set-up to demonstrate the electrolysis of water.
Electrolysis is a chemical reaction caused by passing a direct current (DC) through an electrolyte – a compound which contains ions when molten or in solution. This results in positive ions migrating to the negative electrode (cathode) and negative ions migrating to the positive electrode (anode). The type of electron transfer reaction occurring depends on the electrode potentials of the ions present, and the electrode material may also play a part in the reaction. For example, in the electrolysis of copper salts with a copper anode, atoms of the electrode ionize and enter into solution.
At each electrode the ions are discharged according to Faraday's laws:
1. The quantity of a substance produced is proportional to the amount
of electricity passed.
2. The relative quantities of different substances produce are proportional
to their equivalent weights.
Hence one gram-equivalent of any substance is produced by the same amount of electricity, known as a faraday (96,500 coulombs).
Electrolysis is used to extract electropositive metals, such as sodium, magnesium, and aluminum from their ores, and to refine less electropositive metals; to produce sodium hydroxide (see sodium, chlorine, hydrogen, oxygen, and many other substances.
Development of electrolysis
One of the most influential scientists of all time, Englishman Michael Faraday was fascinated by electricity, magnetism, and the nature of energy and matter. In 1833, Faraday discovered how to reverse the natural process by which electromagnetic bonds are formed. He formulated two laws to illustrate how electrochemistry holds matter together, and described how to manipulate this pattern so that a new arrangement comes about.
Electrolysis requires a liquid medium, the electrolyte, through which a direct electrical current can be passed. The current splits the bond that hold ions in their molecular positions at the anode, or positive end, of the reaction and causes them to enter into new molecular relationships at the cathode, or negative end. The ratio and rate of transfer depend on the nature of the solution, the amount of power used, and the material at the anode, but the process is universal.
Controlling and reversing electrochemical reactions extend humanity's sense of power over the environment. If we could manipulate such fundamental forces, we were not merely Earth's inheritors, we were its masters. Being able to manipulate the very structure of matter at a molecular level enabled us to separate elements from naturally occurring compounds; this massively improved our ability to exploit natural sources of metals, and drove forward industrial and technological expansion. Electrolysis refined our ability to take charge of human progress. It also opened the eyes of the scientific world to the particular nature of matter and the forces that bond it together.


