cross-linking
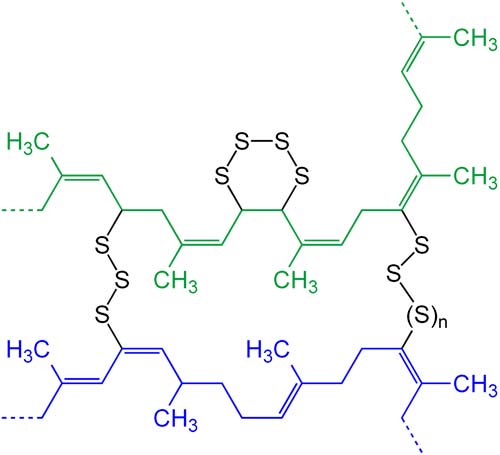
Vulcanization is an example of cross-linking. Schematic presentation of two "polymer chains" (blue and green) cross-linked after the vulcanization of natural rubber with sulfur.
Cross-linking is the attachment of two chains of polymer molecules by bridges, composed of either an element, a group, or a compound, that join certain carbon atoms of the chains by primary chemical bonds. Cross-linking occurs in nature in substances made up of polypeptide chains that are joined by the disulfide bonds of the cysteine residue, as in keratins or insulin.
Cross-linking can be effected artificially, either adding a chemical substance (cross-linking agent), or by subjecting the polymer to high-energy radiation. Examples are: vulcanization of rubber with sulphur, cross-linking of polystyrene with divinylbenzene, or cross-linking of polythene by means of high-energy radiation. Cross-linking has the effect of changing a plastic from thermoplastic to thermosetting. Thus, it also increases strength, heat and electrical resistance, and especially resistance to solvents and other chemicals.


