nucleotide
A nucleotide is a nucleoside to which a phosphate group is attached at the 5' position on the sugar. Nucleotides are organic chemicals of central importance in the chemistry of life. Some provide the basic molecular units for the synthesis of more complex molecules, notably the nucleic acids – DNA and RNA; others – predominantly ATP (adenosine triphosphate) – provide a means of storing and releasing the energy needed to drive biochemical processes.
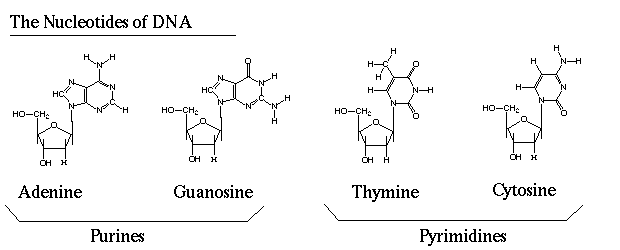
The nucleotide molecule is a three-part structure, comprising a phosphate group linked to a 5-carbon sugar group (pentose) linked in turn to a nitrogenous side group (base). The five commonest bases are the purines adenine and guanine, and the pyrimidines cytosine, thymine, and uracil. Adenine, guanine, cytosine, and thymine serve in the DNA molecules as the four key "letters" of the genetic code. The nucleotides pentose is either ribose or deoxyribose, the latter differing from the former only in having one less oxygen atom. The base-pentose component of a nucleotide is called a nucleoside.
The phosphate group consists of one or a combination of two or three phosphate units, and is accordingly termed a mono-, di-, or triphosphate. The nucleoside adenosine triphosphate (ATP) is a nucleoside consisting of adenine and ribose bonded to a triphosphate group. The importance of ATP as an energy store depends on the third phosphate unit. When a third unit is added to ADP (adenosine diphosphate) to form ATP, an energy-rich chemical bond is formed; and it is this energy, released when ATP is converted back to ADP, that the cell utilizes. By releasing its energy, ATP activates or accelerates the action of enzymes, the catalysts of biochemical reactions, and so belongs to a class of substances called coenzymes. Most coenzymes are nucleotides.


