carbon burning
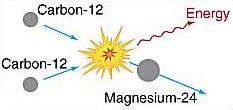
Carbon-carbon fusion.
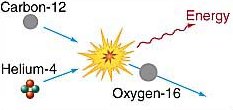
Helium capture.
Carbon burning is the stage at which a star fuses carbon inside its core, making heavier elements such as neon and magnesium. Carbon burning eventually occurs in all stars that start out with more than about eight solar masses.
At a temperature of about 6 × 108 K, carbon nuclei can fuse to form magnesium, as shown in the upper diagram.
However, because of the rapidly increasing nuclear charges (i.e. increasing number of protons in the nuclei), fusion reactions between any nuclei larger than carbon require such high temperatures that they're rare in stars. The formation of most heavier elements occurs by way of an easier path. For example, the repulsive force between two carbon nuclei is three times greater than the force between a nucleus of carbon and one of helium. Thus, carbon – helium fusion occurs at a lower temperature than that at which carbon – carbon fusion occurs. At temperatures above 2 × 108 K, a carbon-12 nucleus colliding with a helium-4 nucleus can produce oxygen-16, as depicted in the lower diagram.
Likewise, one oxygen-16 nucleus may fuse with other oxygen-16 nucleus at a temperature of about 109 K to form sulfur-32, but it's much more likely that an oxygen-16 nucleus will capture a helium-4 nucleus (if one is available) to form neon-20. The second reaction is more probable because it occurs at a lower temperature than that needed for oxygen – oxygen fusion.
So, as the star evolves, heavier elements tend to form through helium-capture rather than by fusion of like nuclei. Because these helium-capture reactions are so much more common, elements with nuclear masses of 4 units (that is, helium itself), 12 units (carbon), 16 units (oxygen), 20 units (neon), 24 units (magnesium), and 28 units (silicon) stand out as prominent peaks in a chart of cosmic abundances. Each element is built by combining the preceding element and a helium-4 nucleus as the star evolves.


