silicon
Figure 1. Silicon.
Figure 2. In the basic unit of crystalline silicon, a silicon atom shares each of its four valence electrons with each of four neighboring atoms.
Silicon (Si) is a dark-gray, metalloid (or semimetallic) element (Figure 1), which is the second most abundant element in Earth's crust (25.7% by mass). It occurs naturally in various forms, including minerals composed of silicates and those, such as quartz, composed of silicon dioxide. Silicon has a diamond-like crystal structure (see Figure 2), although it can also exist in an amorphous state. It is used in its elemental state in alloys and to make semiconductor components.
| atomic number | 14 |
| relative atomic mass | 28.086 |
| electron configuration | 1s22s22p63s23p2 |
| atomic radius | 117 pm |
| oxidation states | 2, 4, - 4 |
| relative density | 2.33 |
| melting point | 1,410°C (2,570°F) |
| boiling point | 2,355°C (4,271°F) |
Chemistry of silicon
Silicon is quite inert at low temperatures, but when strongly heated in air the surface becomes covered with a layer of oxide. Silicon is insoluble in water and resists the action of most acids, but not hydrofluoric. When boiled with alkaline hydroxides, such as sodium hydroxide (caustic soda), sodium silicate is formed:
Si + 2NaOH + H2O → Na2SiO3 + 2H2
Silicon combines with fluorine and chlorine when heated in either of these gases and the corresponding silicon halide is formed. Many organosilicon compounds are known and there has been much speculation over the years on the possibility of silicon-based life.
Silicon carbide
Silicon itself is not very hard, but silicon carbide, known commercially as carborundum, which is obtained by heating a mixture of silica and coke in an electric furnace, is almost as hard as diamond (whose structure it resembles). Carborundum is a black, cubic crystalline solid, which is hard, chemically inactive, and does not decompose until heated to about 2,200°C. Crushed crystals of carborundum can be mixed with a binder, such as clay, and molded into various shapes for use as an abrasive in grindstones and grinding wheels. The blocks and wheels have to be baked subsequently, so that the individual crystals fuse together. Being a good heat conductor, carborundum is also used in making high-temperature bricks.
Silicon dioxide
As silicon lies immediately below carbon, in the same group of the periodic table, we might expect the compounds of these elements to be similar. This is true to some extent, but there is very little in common physically between the oxides of carbon and of silicon. Carbon dioxide is a gas at room temperature, whereas silicon dioxide is a hard solid with a melting point of 1,730°C. Silicon dioxide occurs naturally, as a white or colorless mineral known as silica, as quartz and sand, and in many other familiar rocks and minerals, including flint, granite, amethyst, and rock-crystal. Silica is used in the manufacture of glass, ceramics, concrete, and carborundum.
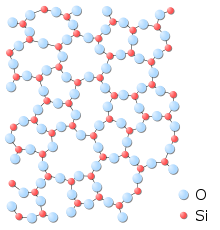 |
| Fig 3. The amorphous structure of glassy silica in two-dimensions.
No long-range order is present; however there is local ordering with
respect to the tetrahedral arrangement of oxygen (O) atoms around
the silicon (Si) atoms. Note that a fourth oxygen atom is bonded to
each silicon atom, either behind the plane of the screen or in front
of it; these atoms are omitted for clarity.
|
The stability of silicon dioxide in its crystalline state stems from its molecular structure. Carbon dioxide, even in the solid state, comprises CO2 units in which two oxygen atoms are joined by double bonds to each carbon. In contrast, the silicon atoms in silicon dioxide are joined by single bonds to four oxygen atoms. The other bond of each oxygen atom is linked to a different silicon atom. Thus each oxygen atom is shared between two silicon atoms. The existence of this macromolecule, as such a giant network of silicon and oxygen atoms is known, accounts for the extraordinary stability of silica.
In addition to the use of sand and various silica-containing stones in the building trade, silica has many other uses, including the manufacture of glass. A special type of glass can be obtained by heating quartz until it melts, and then working the molten mass in much the same way as glass. Quartz glass fibers are used for suspensions in delicate electrical apparatus. Crucibles, evaporating dishes, and similar apparatus made from fused silica are useful for certain types of reactions. At high temperatures silica will combine with bases and metallic oxides to yield silicates, so there is a limit to the reactions for which silica is suitable.
Silicon tetrachloride
Silicon tetrachloride (SiCl4) is a colorless fuming liquid, made by reacting chlorine with a mixture of silica and carbon. It is the starting point for preparing a whole range of silicon-containing organic compounds called silicones.
Silicones
Silicones are polymers, the basic unit of which is a –Si–O–Si– chain of linkages with two organic groups (methyl, ethyl, phenyl, etc) attached to each silicon atom . Most of the silicones are resistant to water and oxidation, and are stable to heat. As they are not wetted by water. they are used for water-proofing or as water repellents. Depending on the structure of the particular molecules, silicones may be oils, greases, or resins. Some are used as lubricants (see lubrication) in situations where there are large temperature variations which would render ordinary oils and greases unsuitable. Silicone rubbers remain flexible at low temperatures.
| Fig 4. Part of a silicone polymer.
|
Silane
Silane (SiH4) is a colorless gas, insoluble in water, which is stable in the absence of air but spontaneously flammable even at low temperatures. Silane itself is the simplest member of the silane family, a series of silicon hydrides, analogous to the alkanes.
| density relative to water | 0.68 (liquid) |
| melting point | -185°C |
| boiling point | -112°C |
Silicosis
Silicosis is a form of pneumoconiosis, or fibrotic lung disease, in which long-standing inhalation of fine silica dusts in mining causes a progressive reduction in the functional capacity of the lungs. The normally thin-walled alveolus and small bronchioles become thickened with fibrous tissue and the lungs lose their elasticity. Characteristic X-ray appearances and changes in lung function appear.


