coordinate bond
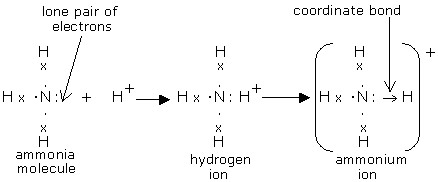
Figure 1. During the formation of an ammonium ion, nitrogen is the donor atom, while H+ is the acceptor ion.
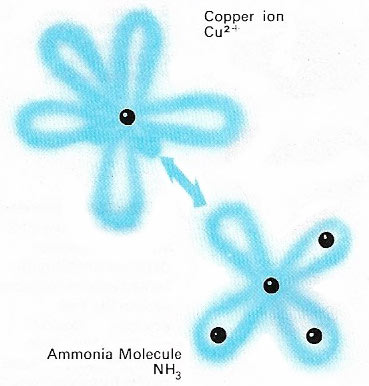
Figure 2. A coordinate bond involves the sharing of two electrons, both contributed by the same combining atom or molecule (hence the alternative name: dative covalency). Here a lone pair of electrons in an orbital of ammonia enter an empty orbital of a copper (cupric) ion to form such a bond with the required number of two electrons. The resulting compounds are called coordination compounds and are often brightly colored.
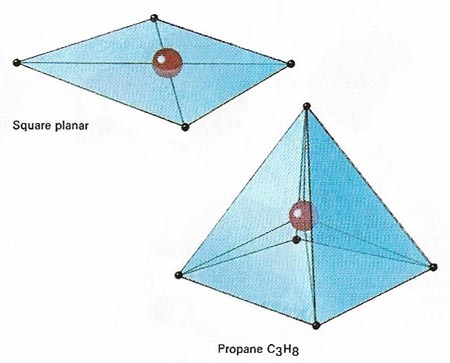
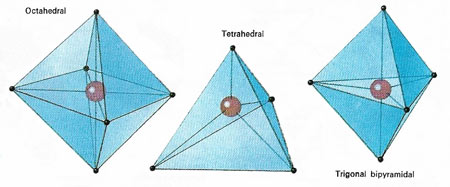
Figure 3. In the case of coordination compounds , in which the central atom is sharing electrons from other atoms and not contributing any of its own electrons to the bonding, the commonly found geometries are those shown above. Whether a compound made of, ay, a central cobalt atom and four outer atoms adopts a square planar or a tetrahedral form depends on the influence of non-bonding electrons associated with the different atoms involved.
A coordinate bond, also known as a dative covalent bond, is the linkage of two atoms by a pair of electrons, both electrons being provided by one of the atoms (the donor). The coordinate bond is formally identical with the covalent bond. An atom capable of accepting the electrons is the acceptor, the molecule donating the electrons is the donor or ligand. Coordinate linkages occur widely in inorganic complexes.
As with any covalent bond, the atoms in a coordinate bond redistribute electrons in order to satisfy the octet rule, which states that each atom will lose, gain, or share electrons in order to have a full valence of eight electrons in its outer shell. Hydrogen is a common exception to the octet rule, and instead follows the duet rule because it only has one 1s orbital and its outer valence is full when it has two electrons.
An example of this is the ammonium ion, NH4+. One of the single bonds between the nitrogen and hydrogen will be a dative covalent bond (Figure 1). Dative covalent bonds have the exact same orbital shapes and repulsion as normal covalent bonds. Ammonium, like methane, would therefore have a tetrahedral shape with bond angles of about 109.5°. Dative covalent bonds are represented on drawings as an arrow, with it pointing towards the atom/ion that isn't donating any electrons to the dative covalent bond.
Coordination compound
A coordination compound, also called a complex compound, is a type of chemical compound in which one or more groups or molecules each form a coordinate bond, usually a transition element (Figures 2 and 3). The complex may be either a complex ion or a neutral molecule, as in nickel carbonyl (Ni(CO)4). Some coordination compounds, such as heme and chlorophyll, have biological importance.
Chelation
Chelation is a chemical reaction in which a certain type of organic compound, known as a chelating agent, combines with a metal ion by forming coordinate bonds with two or more atoms of the organic compound. Examples of chelating agents include tartaric acid (CHOHCOOH)2 and ethylenediamine (CH2NH2)2.
Coordination number
The coordination number is the number of chemical bonds made to a central number in a specified complex salt, such as the number of anions (negative ions) surrounding a cation (positive ion).


