transition element
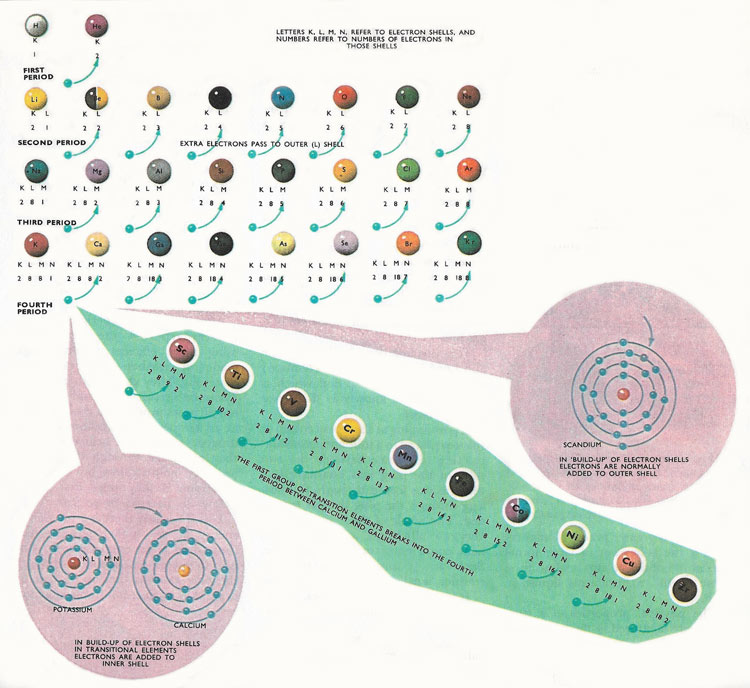
The first part of the periodic table, showing the intervention of the first family of transition elemenets into the fourth period. The electrons are gained by an inner shell, and not the outer shell, as expected.
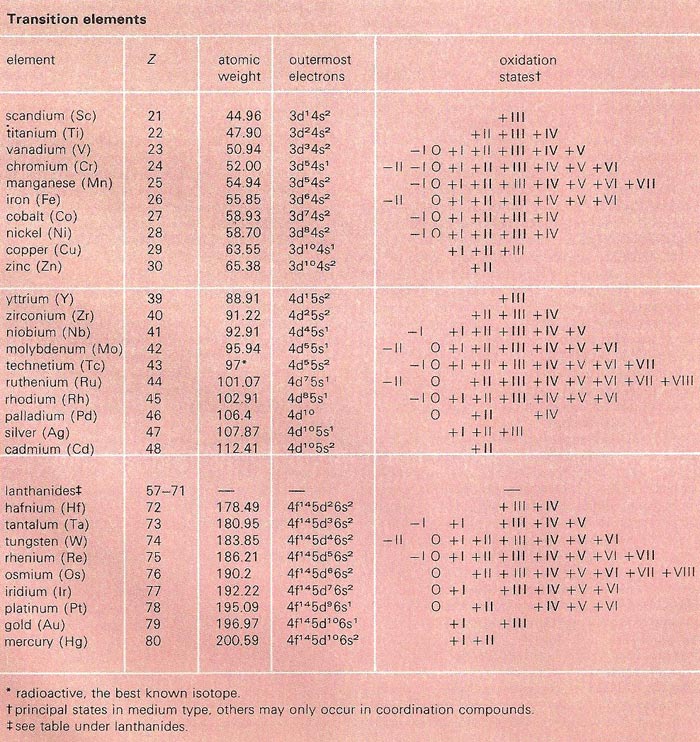

A transition element is a metallic element that has an incomplete inner shell. Transition elements occupy the short groups in the periodic table – i.e., groups IB through VIIB and group VIII – in which the d-orbitals are being filled. In general the transition elements are dense, hard, and of high melting point. Their electronic structures, with many loosely-bound unpaired d electrons, account for their properties: they exhibit many different valence states, form stable ligand complexes, are mostly colored and paramagnetic, and are generally good catalysts. They form many stable organometallic compounds and carbonyls (compounds in which carbon monoxide, CO, acts as a ligand) with specially stable "push-pull" bonding. The second and third row transition elements are less reactive than the first row, and stable in higher valence states. Many transition elements are technologically important.
Historical introduction
There are 92 naturally occurring elements and almost two dozen heavier, artificial elements. The properties of the elements differ greatly, ranging from highly reactive gases such as fluorine and chlorine and highly reactive metallic solids such as sodium and potassium to the inert gases and the heavy unreactive metals such as silver and gold. Since the earliest days of chemical science, attempts had been made to put some sense and order into the classification of the elements – to explain the wide differences in the properties of some elements and the similarities of others. This was eventually done, with outstanding success, by Dmitri Mendeleyev when he devised his periodic classification. He found that if the elements were arranged in order of increasing atomic weight, they fell into natural periods. The two lightest elements, hydrogen and helium, form a short period of their own. These are followed by the period of eight elements starting with lithium and ending with neon. The next period of eight elements starts with sodium and ends with argon.
When the elements were arranged in this way, they formed natural families. In each period there was a gradual change in properties from one element to its neighbor. In the first period, the first member, lithium, loses an electron very easily – in other words, it is strongly electropositive. Proceeding through the period, the elements become less electropositive until the seventh member is the strongly electronegative fluorine. The eighth member of the period, neon, is almost totally inert. In the second period, the change in properties is repeated. The first member, sodium, is very similar to lithium; the second member, magnesium, is very similar to the second member of the first period, beryllium. This repetition of properties carries on through the second period, and the periods that follow it.
Although Mendeleyev did not realize it at the time, he had arranged the elements according to the numbers of electrons each element has in the outer shell of its atom. Thus, lithium and sodium each have one electron in their outer shells and are very similar chemically to the first member of the third period, potassium, which also has only one electron in its outer shell. Moving along each of the early periods there is a build-up in the number of electrons in the outer shells of the atoms. When the last, inert element (neon, argon, etc.) is reached there are eight electrons in the outer shell.
All the chemical properties (and many of the physical properties) of the elements are decided by the arrangements of the electrons in the shells. The outer shell is particularly important because it contains the valence electrons. These are the electrons that can be given to other atoms to form electrovalent bonds, or shared with other atoms in covalent bonds. The number of electrons in the outer shell is the main factor that decides what sorts of chemical reactions the element undergoes and how it becomes bonded to other atoms. So, by arranging the elements in the periodic classification, Mendeleyev automatically sorted the elements into groups with equal numbers of 'outer shell' electrons. This also meant that the elements were classified according to chemical properties.
The 'octave' classification with the number of electrons increasing until the 'magic number' of eight electrons is achieved works very well for the elements of small atomic weight, but it falls down in the fourth period of elements. In the earlier periods there is the expected build up of electrons in the outer shell, from one to eight, but in the fourth period this pattern is interrupted. The first element, potassium, has a single outer shell electron. The second element, calcium, has tow electrons. The third element, scandium, would be expected to have three electrons, but, in fact, has only two. Most of the next nine elements in the third period have only two electrons in the outer N shell. The atoms of the elements have extra electrons but instead of going to the outer shell they are going to the next inner M shell. So scandium has only two electrons in the N shell, but has nine in its M shell. Its neighbor titanium has ten electrons in its M shell, and the build-up of electrons in the M shell continues until zinc is reached. This has eighteen electrons in its inner M shell. After zinc, the original pattern, with the build-up of the outer N shell of electrons is continued. The last elements of the period all have eighteen electrons in the M shell and they progress, one electron at a time until eight electrons are acquired by the outer N shell.
All the elements from scandium to zinc are called transition elements. In the fifth and sixth periods of the periodic table the build-up of the outer shell is similarly interrupted. Further families of transition elements are formed by the build-up of electrons in inner shells in these periods. The outer shells contain only two electrons and the elements have many properties in common.
Properties of the transition elements
Many of the most common and important metals are transition elements, including iron, copper, zinc, silver and gold. All of the transition elements are dense shiny metals – good conductors of heat and electricity. Included in the transition metals are the strongly magnetic (ferromagnetic) metals iron, cobalt and nickel. Most of the other transition metals are paramagnetic – weak versions of the three magnetic materials. These magnetic properties are due, once again, to the arrangements of electrons in the shells. In ferromagnetic materials, for example, there are unpaired electrons in the shells – for one electron spinning in one direction there is not another spinning in the opposite direction to neutralise the magnetic effects produced by the first.
The chemical properties of these metals are largely affected by the tendency of the electrons in the inner shell to take in chemical bondings. Apparently, there is an 'urge' on the part of these electrons to behave in a similar way to the valence electrons in the outer shell. They are similar in be ha vi our to the electrons in the outer shell because they, too, are not occupying a complete shell of electrons. For example, an atom of the transitional element iron may lose two electrons from its outer shell to form the ion Fe++, or, in addition, lose a third electron from the next shell to form the ion Fe+++. The main factor in determining the chemical properties of these elements is, however, the electrons in the outer orbit, and in passing along the period from one element to the next the properties of neighboring transitional elements change very little.
The transitional elements all form positive ions, usually highly colored both in the solid and in solution. Some of them also form complex ions. The metallic ion takes up molecules of water or ammonia, or even other ions and chemical groups (e.g. the cyanide ion CN–) to form these coordination compounds. A well known example of this is the cuprammonium ion (Cu4NH3)22+ formed when ammonia solution is added to a solution of a copper salt. The deep-blue solution that results is used to confirm the presence of copper ions in qualitative analysis.
| Table of transition elements | ||
|---|---|---|
| element name | symbol | atomic no. |
| Scandium | Sc | 21 |
| Titanium | Ti | 22 |
| Vanadium | V | 23 |
| Chromium | Cr | 24 |
| Manganese | Mn | 25 |
| Iron | Fe | 26 |
| Cobalt | Co | 27 |
| Nickel | Ni | 28 |
| Copper | Cu | 29 |
| Zinc | Zn | 30 |
| Yttrium | Y | 39 |
| Zirconium | Zr | 40 |
| Niobium | Nb | 41 |
| Molybdenum | Mo | 42 |
| Technetium | Tc | 43 |
| Ruthenium | Ru | 44 |
| Rhodium | Rh | 45 |
| Palladium | Pd | 46 |
| Silver | Ag | 47 |
| Cadmium | Cd | 48 |
| Tantalum | Ta | 73 |
| Tungsten | W | 74 |
| Rhenium | Re | 75 |
| Osmium | Os | 76 |
| Iridium | Ir | 77 |
| Platinum | Pt | 78 |
| Gold | Au | 79 |
| Mercury | Hg | 80 |
| Rutherfordium | Rf | 104 |
| Dubnium | Db | 105 |
| Seaborgium | Sg | 106 |
| Bohrium | Bh | 107 |
| Hassium | Hs | 108 |
| Meitnerium | Mt | 109 |
| Uununnilium | Uun | 110 |
| Unununium | Uuu | 111 |
| Ununbium | Uub | 112 |


