hydrochloric acid
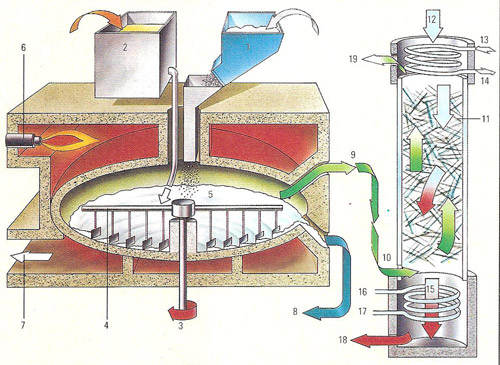
Figure 1. Manufacture of hydrochloric acid using a Manheim type furnace.

Figure 2. A demonstration of the reaction between hydrochloric acid and ammonia resulting in the production of white clouds of ammonium chloride (NH4Cl).
Hydrochloric acid (HCl) is a clear, colorless, fuming, poisonous, highly acidic, aqueous solution of hydrogen chloride, HCl. A saturated solution of hydrochloric contains about 43% HCl and gives a constant-boiling mixture. It is an extremely corrosive mineral acid and must be handled in glass or plastic equipment or in apparatus using special alloys (tantalum, nickel-molybdenum).
Hydrochloric acid is used in petroleum production, as a chemical intermediate, in ore reduction, food processing, pickling, and metal cleaning. It was formerly known as 'spirits of salt'.
In all manufacturing processes for hydrochloric acid another useful product is obtained along with the acid. In the process illustrated (right), using a Manheim type furnace, sodium sulfate is produced.
Key to Figure 1
1) Common salt is added to the furnace.
2) Sulfuric acid inlet by way of a lead-lined tank.
3) Rotating shaft.
4) Rotating stirrers mix reactants.
5) Reaction chamber. The salt and sulfuric acid react to form sodium sulfate
and hydrochloric acid, which comes off as the gas hydrogen chloride because
of the high temperature.
6) Oil burner heats reaction chamber.
7) Combustion gases outlet.
8) Salt cake (sodium sulfate) outlet.
9) Hydrogen chloride gas led off.
10) Hydrogen chloride gas piped into the absorption column below the packed
section.
11) The absorption chamber is packed with Raschig rings made of glass. On
the surface of these rings the hydrogen chloride combines with water, emitted
at the top of the tower (12), to form hydrochloric acid. This reaction releases
heat.
12) Water inlet. The water passes down the packed column and dissolves the
hydrogen chloride gas.
13) Cooling water inlet.
14) Cooling water outlet.
15) Hot concentrated hydrochloric acid passes into the cooler at the bottom
of the column.
16) Cooling water inlet.
17) Cooling water outlet.
18) Cool hydrochloric acid led out to storage tanks.
19) Spent gas vent.
Stomach acid
Dilute hydrochloric acid is produced by the stomach lining and is important in the digestion of proteins. Excessive acid production, which may be stimulated by stress or tobacco smoking, results in the condition known and hyperchlorhydria and may cause gastric ulcers. In acid reflex (backflow of stomach acid into the esophagus), hydrochloric acid may cause esophagitis and heartburn.


