polar molecule
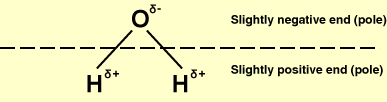
Water: a well-known polar molecule.
A polar molecule is a molecule in which there is some separation of charge in the chemical bonds, so that one part of the molecule has a slight positive charge and the other a slight negative charge, i.e., the molecule has a permanent electric dipole (a pair of equal and opposite charges a short distance apart). Water is a well-known example of a polar molecule.
A polar molecule forms when an atom of high electronegativity (one that attracts electrons), such as chlorine, bonds with a less electronegative atom such as hydrogen. Because the more electronegative atom pulls the electron(s) away from the other atom, the molecule formed has one end which is negatively charged and another which is positively charged. Polar molecules tend to align themselves because the negative end of each molecule is attracted to the positive end of other molecules, and vice versa.
Polar molecules containing hydrogen are so strongly polar (and therefore attracted to each other) that they form a type of chemical bond called a hydrogen bond.


