acid chloride
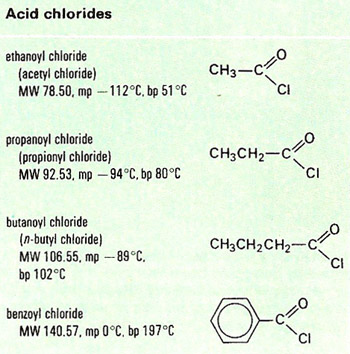
An acid chloride is a member of a class of organic compounds with the general formula RCOCl.
Acid chlorides, or acyl chlorides, are prepared by reacting carboxylic acids with phosphorus (III or V) chloride or thionyl chloride (SOCl2). They are volatile, fuming, pungent liquids, corrosive and very reactive. They react with alcohols to give esters, with ammonia and amines to give amides, and with water to give carboxylic acids.
Acid chlorides are reduced to ketones by Grignard reagents and in the Friedel-Crafts reaction. Other acid halides are similar. See also aldehydes and acid anhydrides.


