Is there life on Titan?

False-color view of Titan made from a number of images captured by Cassini's infrared camera, which can penetrate some of Titan's clouds. Image credit: NASA/JPL.

The surface of Titan, as imaged by the Huygens probe. Credit: ESA/NASA/JPL/University of Arizona.
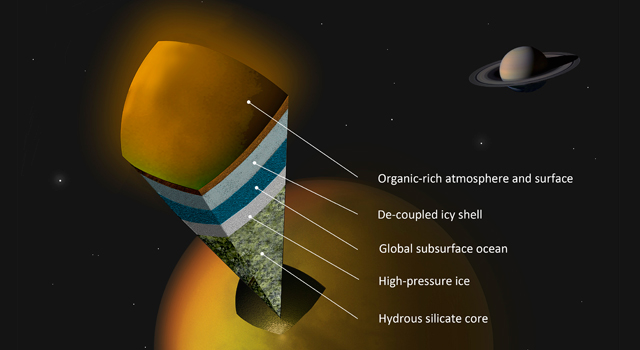
Artist's concept of a possible scenario for the internal structure of Titan. Image credit: A. Tavani.

Pitch Lake, Trinidad.
In many ways, Titan is the most unusual and exotic world we know. It is by far the largest moon of Saturn and, for many years, was thought to be the largest moon in the solar system, though Ganymede of Jupiter has now replaced it at the top of that league. It is also the only moon in the Sun's kingdom to have a dense atmosphere; in fact, the surface pressure on Titan, at 1.5 bars, is 50% greater than on our own world. And Titan's atmosphere is the only one known, other than Earth's, of which the main ingredient is nitrogen (about 95%), the rest being mostly methane with an eclectic sprinkling of organic chemicals. A similar atmospheric composition is believed to have existed on Earth around the time terrestrial life began, and has led to speculation over the years that Titan may be in an early and possibly arrested stage of pre-biological development (see Titan, prebiotic evolution).
The surface of Saturn's giant moon was a complete mystery until quite recently, obscured as it is by a permanent haze of orange smog. But our knowledge of Titan expanded dramatically with the arrival of the Cassini spacecraft in June 2004, and the extraordinary success of the Huygens probe, which was released by Cassini and parachuted safely down on to Titan's surface. For two hours, Huygens beamed back remarkable images and other scientific data from its landing spot amid a wasteland of ice and frozen hydrocarbons over a billion kilometers away.
Strange new world
It doesn't rain water on Titan. Images from Chile's Very Large Telescope and Hawaii's Keck Observatory in 2005 and 2006 showed for the first time nearly global cloud cover at high elevations on the moon, from which a persistent morning drizzle fell over the western foothills of Titan's major continent, Xanadu. It was a drizzle of methane.
On our planet we're used to thinking of methane as a gas, but on Titan, because of the low temperatures, this simplest of hydrocarbons is the main liquid present. In place of a terrestrial water cycle, Titan has a methane cycle. But the methane that rains down from Titan's skies rarely reaches ground level. Instead, it usually evaporates above the surface, just as rainwater often does over the southwestern desert of the United States. Only very occasionally, it seems, does Titan experience a real downpour. According to Ralph Lorenz of the Applied Physics Laboratory at Johns Hopkins University, these methane monsoons occur maybe once every few centuries and last for months.
Some features on Titan look strikingly Earth-like but are the work of a distinctly non-terrestrial chemistry and weather system. Large parts of the big moon are marked by what appear to be river channels and their tributaries which, though probably dry much of the time, must have been carved by running methane. Certain latitudes on Titan are dominated by dune fields; others, around the poles, are pockmarked by numerous lakes, large and small, filled with liquid hydrocarbons – primarily methane and ethane (the second simplest hydrocarbon) with some nitrogen mixed in. Steep valleys and towering cliffs in many places make the moon's landscape rugged, and slick ice would add to a visitor's perils. By contrast, the Huygens probe landing site was relatively flat and desert-like, its terrain broken only by a plethora of small, ice cobbles.
With temperatures at ground level hovering around –180°C, the prospects for liquid water on or near the surface of Titan might seem non-existent. Yet there is some evidence for running water in the form of bright flows, quite unlike the dark ones of liquid hydrocarbons. Low-temperature volcanic activity, or cryovolcanism, powered by tidal forces from Saturn, is the likeliest explanation for once-moving water.
Activity of a different kind takes place in Titan's dense atmosphere, driven by ultraviolet radiation from the Sun and, to an even greater degree, by radiation accelerated in Saturn's powerful magnetic field. The high-energy bombardment promotes the assembly of organic chemicals by splitting many of the compounds in the atmosphere into radicals – molecular fragments that have a free electron and are very reactive. One of the organic materials made in this way is acetylene, an energy-rich hydrocarbon compound containing two carbon atoms, joined by a triple bond, and two hydrogen atoms. Acetylene forms in Titan's atmosphere as solid particles, which then settle onto the moon's surface. Under normal conditions on Earth, acetylene is explosive and has to be handled with great care. However, in Titan's cold surface environment, in which reactions would otherwise be painfully slow, it's an ideal substance for promoting the build-up of complex molecules.
Titan's garden
Scientists have just begun to take seriously the possibility of life on Titan, either on or below the surface. Speculation about an internal ocean, similar in nature to those suspected on some of Jupiter's moons, has been given a boost by recent data from Cassini. If Titan does have an ocean, its waters are likely to contain a hefty dose of ammonia, thanks to the high abundance of nitrogen on the moon. This would serve as an antifreeze, keeping the ocean liquid at much lower temperatures than if it were pure water – down to nearly –100°C.
In general, it seems that if life does exist on Titan, it must be very different from anything we know on Earth. A combination of extreme cold and strange, hydrocarbon-based chemistry would virtually ensure a unique set of adaptations, from the biochemical level on up. Moreover, a separate origin of life would be almost guaranteed because of the extreme unlikelihood of organisms being transported on meteorites between the inner and outer solar system. The discovery of "weird life" and a second genesis on Titan would have far-reaching implications for the abundance and diversity of life throughout the universe.
Life on Saturn's big moon would be alien in many respects, yet it might still use some of the same tricks that terrestrial animals and plants turn to in tough times. Take, for example, eastern skunk cabbage. Symplocarpus foetidus, to give it its scientific name, is a strange plant with smelly leaves which belongs to a group of thermogenic or "heat-generating" species. Remarkably, it produces enough heat to lift its temperature 15°C to 35°C above that of the surrounding air, enabling it to melt its way through frozen ground before flowering. A talent like this, adapted to work at even lower temperatures, would be ideal on Titan, where a creature might use chemistry to make its immediate environment more habitable.
In fact, many types of Earth organisms living in frozen surroundings, even algae, give off some waste heat from their metabolic reactions that can be used to melt their own little watering holes in ice. Such holes are commonly seen on glaciers and in Arctic pack ice and are known as croconites. Some of the meltwater underneath the glacial ice sheets even releases methane. Could such a mechanism explain both the apparent smoothness and youthfulness of Titan's surface and the high methane levels in the atmosphere?
Titan's methane has always puzzled scientists. In the moon's lower atmosphere the concentration reaches about 5%, yet there is no obvious source for so much of this gas. The traditional explanation is that a lot of methane becomes trapped in Titan's icy crust over the eons and is now gradually being released. But it's hard to understand, in purely chemical terms, how this vast store of methane could have built up, given that the gas is continually broken down in the moon's atmosphere (by a process similar to, but slower than, that occurring on Mars). On Earth, methane is usually an end product of metabolism by microbial organisms, and the same may well be true on Mars.
One possibility is that organisms in Titan's icy crust free the high-grade energy stored in acetylene by catalyzing the reaction with hydrogen, another constituent of the moon's atmosphere, to form methane. Each gram of acetylene exploited in this way could supply about 3.85 kilojoules (nearly 1,000 calories) of energy. Given the current level of methane in the atmosphere, and the rate of destruction of the gas, calculations show that about 1021 kJ (one billion trillion kJ) would have been released by acetylene-hydrogen reactions over the past ten million years.
One of the few life-forms on Earth for which the amount of energy needed to make new individuals is reasonable well known is the yeast Saccharomyces cerevisiae. Making a gram of this yeast "from scratch" takes about 3.2 kJ. Assuming that any microbes on Titan use a similar amount of energy to build their bodies, and that all of Titan's methane is produced biologically (a big "if"!), it's possible to work out the average concentration of living matter on Saturn's largest moon. The figure that emerges corresponds to just over ten million terrestrial-sized organisms per millimeter – on a par with what would be expected of a slightly nutrient-poor environment on Earth. The density of living material almost certainly wouldn't be uniform over the whole moon, but would vary according to local availability of acetylene and other nutrients.
Based on these calculations, an assessment can be made of whether biological heating– thermogenesis – is a viable option on Titan. The energy-making acetylene reaction mentioned above would yield about 3.85 kJ/g. If organisms on Titan had the same energy needs as S. cerevisiae, they'd have about 0.65 kJ/g left over which could be expelled in the form of heat. This, in turn, could melt a substantial amount of ammonia-water ice – close to 100 million metric tons per year.
Of course, all these calculations and estimates have wide margins of error and are, by necessity, based on assumptions about Earth-like microbiology and biochemistry. But, in fact, these assumptions are conservative because anything living on Titan would be far better adapted to local conditions than any terrestrial microbe – for example, it would probably be very efficient in its use of energy. Whatever the detail, a number of key facts come out of this analysis: biochemical heating on Titan is a distinct possibility; the proposed reaction with acetylene is a reasonable metabolic pathway; and the production of methane, if biological, suggests the existence of a large population of organisms on the moon.
Aliens of Pitch Lake
Astrobiologists are always on the look out for analog sites. These are places on Earth that have some features in common with extraterrestrial locations. Some parts of the Atacama Desert and Antarctica, for example, are Mars-like. The question is whether any analog site exists for Titan.
It turns out there are two of them, one in Trinidad, the other in Venezuela. Both are natural lakes of asphalt – a thick liquid mixture of hydrocarbons.
The Trinidadian site, known locally as Pitch Lake, has been used as a source of asphalt worldwide. Large parts of New York were paved with material from the lake. As visitors discover, the asphalt is mostly firm, like congealing lava, and only the active, upwelling areas are truly in a liquid state. Occasionally unwary trekkers get struck in the soft spots and have to be rescued.
Scientists wanting to take samples from the liquid areas of Pitch Lake have to contend with the very gooey nature of the asphalt. It quickly and persistently sticks to skin and anything else with which it comes into contact. The temperature of the liquid patches is about 30°C – typically a few degrees warmer than the surrounding air – demonstrating clearly that there is an underground heat source.
Gas bubbling out of the asphalt contains methane in low but significant concentrations. This and other hydrocarbons have presumably made their way into Pitch Lake from offshore oil reservoirs. Could their conceivably be anything alive in such a dark, syrupy brew? Surprisingly, yes. DNA sequencing and other methods of molecular analysis have confirmed that many microbes make a living in this extraordinary environment, most of them producing methane as a by-product of their metabolism. By an astounding coincidence, the concentration of biological matter turns out to be roughly ten million organisms per milligram of asphalt – in the same ballpark as our early estimate for the density of life on Titan.
Even more remarkable is that some microbes live in Pitch Lake in the virtual absence of water. There's in a quantity in science known as water activity, which tells how much "free water" is available for use in chemical reactions or by living things; it excludes any water that's already tied up with other substances or on surfaces. In pure, fresh water the water activity is 1.0. No organisms have been confirmed to date in an environment where the water activity is less than 0.6. However, when a sample of the contents of Pitch Lake were tested as part of a Discovery Channel documentary, a water activity of 0.49 was found in regions of the lake from which microbial samples for DNA analysis had previously been taken. If this result is upheld by other methods, it would mean that some terrestrial life can metabolize in an almost waterless environment and, just possibly, may not use water as its biological solvent at all, but a mixture of hydrocarbons.
Pitch Lake is certainly not an exact analog of Titan, most obviously with regard to temperature. However, the Trinidadian asphalt and the organisms found in it show that life can adapt to being permanently bathed in various oils and will hopefully teach us in the long run how their evolution came about. Future research will also show what mechanisms and biochemical changes these organisms employ, and whether perhaps their adaptations would be useful to survival on Saturn's giant moon.
Cool life
The reaction of acetylene with hydrogen to produce methane isn't the only one that could supply enough energy for a metabolic pathway on Titan. Chris McKay from NASA Ames and Heather Smith from the International Space University in Strasbourg, France, have suggested that heavier hydrocarbons might also serve as a raw fuel for life.
Another possibility involves high-energy radicals. Carbohydrate and nitrogen radicals, for example, could react to form the compounds hydrocyanic acid and cyanamide. On Earth, radical reactions are rarely used in metabolism because they're so difficult to control and can cause internal damage to organisms, ripping cells apart like miniature explosives. But in the super-cold conditions on Titan, these reactions would proceed much more slowly and be biologically manageable. What's more, hydrocyanic acid and cyanamide are thought to have been important ingredients in the origin of life on Earth. Hydrocyanic acid, consisting of one hydrogen, one carbon, and one nitrogen atom, forms amino acids when exposed to ultraviolet radiation. Cyanamide is a compound used in terrestrial biochemistry to help bind amino acids together to form proteins.
Differences in biochemistry suggest differences also in the makeup of cells. On Earth, the membrane of a cell, through which a simple organism interacts with its environment, consists basically of a double layer of lipid (fat-like) molecules. The orientation of these molecules is quite specific, due to the fact that water – the solvent of life on our world – is polar. In other words, a water molecule has a slight positive charge at one end and a slight negative charge at the other. The lipid molecules in terrestrial cells each have a "water-loving" polar head which points outward, and a "water-hating" non-polar tail which points inward. Only because of this arrangement can life-as-we-know-it interact with water and extract nutrients from it. If life on Titan uses hydrocarbons, such as methane and ethane, as its solvent of choice, then its cellular architecture must be reversed or non-polar at both ends. Most hydrocarbons are non-polar so that for an organism to interact with them successfully it would need the non-polar ends of its cell membrane molecules pointing to the outside.
Silicon and life
Cell membranes on Titan might be unusual in other way – by incorporating silicon compounds called silanes in their construction. Silane itself (the silicon analog of methane) contains one silicon and four hydrogen atoms in a tetrahedral structure, and is a reactive gas under normal Earth conditions. For example, it will immediately combine with water and oxygen to form silicon dioxide, the substance of which sand and quartz are made. However, the environmental circumstances on Titan are vastly different. Oxygen and liquid water are nearly absent, and even carbon dioxide is very rare. Francois Raulin from the University of Paris has suggested that life on Titan might compensate for the lack of available oxygen by using nitrogen analogs in its biochemistry.
Silanes have a number of properties that would make them biochemically attractive in the right setting. Silane itself would be a liquid under Titan surface conditions, but polysilanes, consisting of many silane molecules stacked together, would form solids at those temperatures. William Bains from the University of Cambridge Institute of Biotechnology has even come up with a model for photosynthesis based on silicon biochemistry.
Given the abundance of carbon on Titan and carbon's unparalleled ability to form and break bonds in complex substances, it's unlikely that actual silicon organisms exist on the big moon. Silicon-based life is a staple of science fiction. But although Titan probably lacks anything like the rock-boring Horta, featured in one of the original Star Trek episodes, it may well find novel biochemical uses for this element.
Strangely familiar
The size of living cells on Earth may be a misleading model for life in an environment such as on Titan, which is not dominated by water, a highly polar and relatively high-temperature solvent. As pointed out by Louis Irwin from the University of Texas at El Paso, in an extremely cold, non-polar, liquid hydrocarbon environment, it's possible that life consists of much larger cells, perhaps even visible to the unaided eye. Given the cold surface temperatures, metabolism may proceed very slowly, however. The lifespan of humans and most other organisms on Earth is less than a century, but there is no reason why lifespans on Titan might not be measured in thousands or hundreds of thousands of years.
Although any life on Titan might have a quite unfamiliar biochemistry, it would not be impossible to detect. To be effective, a search for such life would have to take a broader approach than screening for specific molecules, like DNA, ATP, or chlorophyll, which are central to terrestrial life. A life-searching probe on Titan would need to look for any complex organic compounds, especially those of high molecular weight (500 or greater). This is because we expect all life, whatever its origin, to depend on large, highly-specialized molecules. There are also good reasons to suppose that even life very different from our own would use biomolecular building blocks of a particular handedness. For example, all life on Earth uses right-handed sugars (in DNA, RNA, and metabolic pathways) and left-handed amino acids within proteins. Life elsewhere might differ in its preferences for right-handed or left versions of whatever molecules it depends on, but the chances are it will have a preference, which robotic instruments could be designed to detect.
Scientists are also aware of other likely signatures of life in general. For example, it's reasonable to assume, even in alien microbes, the presence of compartments and boundaries within a cell and from the outside environment to ensure that an organism can operate and maintain itself as an independent entity. A boundary layer is also needed in order to regulate the intake of food from the environment and prevent the intake of food from the environment and prevent the uptake of harmful substances. Its nature may be quite different from cell membranes used by living things on Earth, but it would serve the same essential purpose.
The metabolism of Earth organisms is based on an intricate interplay of reduced and oxygenated compounds, in other words compounds that have either a surplus or a deficiency of electrons. Astrobiologists suspect this may be a universal condition of life. Since Titan's environment has a huge over-abundance of reducing chemicals, the discovery of oxygenated compounds in a high enough quantity might indicate the presence of chemical reactions mediated by life.
Until recently, scientists tended to shy away from talking about actual biology on Titan, preferring to entertain only the possibility of pre-biological chemistry. But new findings about the moon by the Cassini spacecraft have encouraged bolder statements. For example, a report on the limits of organic life in planetary systems published in 2007 by the National Academy of Sciences pointed out that Titan has the basic requirements for life, including disequilibrium conditions, abundant carbon-containing compounds, and a fluid environment. "This makes inescapable," it said, "the conclusion that if life is an intrinsic property of chemical reactivity, life should exist on Titan." Future missions to Saturn and its giant moon will put this intriguing assessment to the test.

