explosives

Figure 1. Left: High explosive for blasting rock. Right: Detonator.

Figure 2. Propellant explosive.
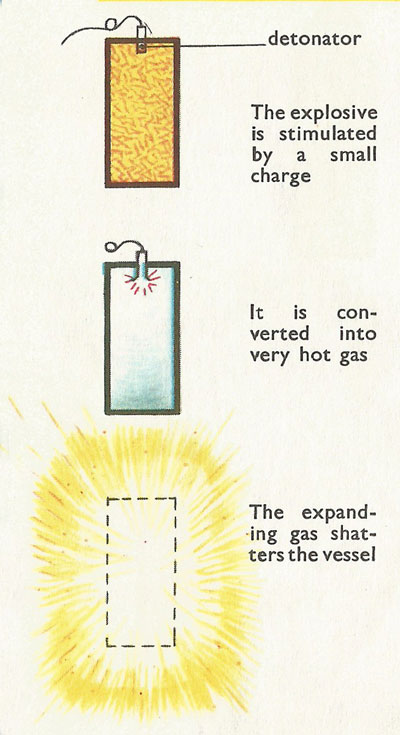
Figure 3. The three stages of an explosion.

Figure 4. Firework.
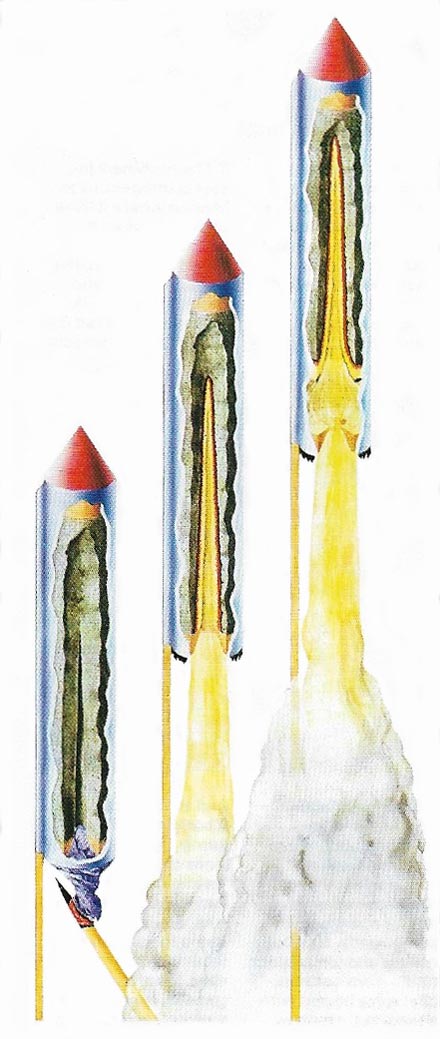
Figure 5. The combustion of the propellant in a firework rocket produces its thrust. The case is wet-rolled and the thrust increased by constricting it near one end (constriction made with cord before the case is dry). The propellant is packed into the case so that a conical cavity is formed at the burning en, allowing a large surface area of combustion to give the initial push. The cap of the rocket contains flares, or 'stars', which are ejected as the propellant burns out.
Explosives are substances capable of very rapid combustion (or other exothermic reaction) to produce hot gases whose rapid expansion is accompanied by a high velocity shock wave, shattering nearby objects. The detonation travels 1,000 times faster than a flame.
The earliest known explosive was gunpowder, invented in China in the tenth century AD, and in the West by Roger Bacon (1242).
Explosives are classified as primary explosives, which explode at once on ignition, and are used as detonators; and high explosives, which if ignited at first merely burn, but explode if detonated by primary explosion (Figure 1). The division is not rigid. Military high explosives are usually mixtures of organic nitrates, TNT, RDX, picric acid, and PETN, which are self-oxidizing. Commercial blasting explosives are less-powerful mixtures of combustible and explosive substances; they include dynamite (containing nitroglycerin, ammonium nitrate, and sometimes nitrocellulose), ammonals (ammonium nitrate + aluminum), and Sprengel explosives (an oxidizing agent mixed with a liquid fuel such as nitrobenzene just before use). Obsolete explosives include the dangerous chlorates and perchlorates, and the uneconomical liquid oxygen explosives (LOX). Explosives which ignite firedamp are termed "permissible," and may be used in coal mines. Propellants for guns and rockets are like explosives, but burn fast rather than detonating.
Fireworks
Fireworks are combustible or explosive preparations used for entertainment, probably first devised in ancient China to frighten off devils. Their initial European use was as weaponry and not until after about 1500 were they employed for entertainment.
Compounds of carbon, potassium, and sulfur are the prime constituents in fireworks, colors being produced by metallic salts (e.g., blue, copper; yellow, sodium; red, lithium or strontium; green, barium), sparks and crackles by powdered iron, carbon, or aluminum, or by certain lead salts (Fig 4).
History of explosives
One day in 1913 when the Canadian Northern Railway's tunnel was being built at Yale, British Columbia, an engineer made a mistake in the strength of a dynamite blast that he had ordered. Instead of merely breaking loose a calculated amount of rock it knocked the entire face of a cliff into the Fraser River. It was the time when the salmon were coming upstream to breed. The rock caused a dam 9 ft high on the river, and the salmon could not jump it because the water was coming over the top with the strength of a fire-hose. It is estimated that millions of salmon died because of the engineer's mistake.
Four years later, on December 6 at Halifax, Nova Scotia, a French munitions ship blew up in the harbour, destroyed a large part of the city and killed 1,600 people – one of the worst explosions in history.
These stories illustrate the two main uses of explosives: in the first they were being used to help man's progress, and in the second they were intended to be used as a weapon or war; but both also show that human errors (and these were made by experts) can cause fantastic harm and devastation.
The conclusion is obvious: while explosives are one of the most important inventions of mankind and have changed the whole course of history, they are also one of the most dangerous.
Handling of explosives
Governments are quite aware of the danger of explosives, and in Britain the Explosives Acts of 1875 and 1923 limit the making of explosives to special factories. Buildings have to be built away from each other and some have to be surrounded by a protective embankment. A typical factory is divided into 'non-danger' and 'danger' areas. The manufacture of nitric and sulfuric acids takes place in the non-danger area. The explosives are made and packed in the danger area.
The main danger is ignition by spark, friction, or impact. Naked lights, steel tools, or anything that might produce a spark or flame are excluded from the building. Each factory had a 'clean' floor, and workers can only walk on it in specially cleaned shoes.
Propellants and high explosives
The two main types of explosives are propellants and high explosives. The propellant, as its name implies, has a pushing action and the high explosive a shattering or blasting effect. Modern propellants such as cordite are made by mixing nitroglycerine and nitrocellulose (guncotton) and are used in cartridges for all kinds of guns and sometimes for starting aircraft and diesel engines. Propellants are also used for guided missiles and rockets.
Gunpowder, the oldest and easiest explosive to make, can be ignited by a spark or a flame, but with the improved types used today other methods are employed. Propellants can be set off by a flame coming either from a small quantity of explosive held in a cap (when you pull the trigger of a gun the cartridge cap is hit sharply) or from a gunpowder igniter. This consists of a thin wire embedded in gunpowder: an electric current is passed through the wire which heats it so that it sets light to the powder. The most commonly used explosive is TNT (trinitrotoluene). Nearly all high explosives are made by treating some substance containing carbon with nitric acid.
High explosives must be set off by a violent shock, as they will just burn without exploding if a flame is applied. Detonators containing an explosive charge which can be easily set off, such as cyclonite, tetryl or mercuric fulminate, are used for this purpose. Some artillery shells contain high explosive. A detonator is fitted on the nose of the shell so that when the shell hits its target the high explosive is set off, causing widespread damage.
Detonator
A detonator is a small explosive device used to initiate the explosion of a main explosive charge in quarrying, mining, or tunneling operations and in ammunition. Safer, i.e., less sensitive, explosives need stronger detonators. The typical detonator comprises a thin-walled metal or plastic tube containing a charge of mercury fulminate (Hg(ONC)2) or, now more common, lead azide (PbN6), often mixed with other chemicals. The detonator is set off by a flame from a safety fuze or electrically, by passing a current through an attached resistance wire.
Developments in manufacture
The first true gunpowder is believed to have been made by the English Friar Roger Bacon in the thirteenth century, although it is known that the Chinese and the Arabs had produced similar mixtures several centuries earlier. Soon after the invention of gunpowder, its military uses were appreciated and new weapons were made. From that time onwards explosives had a great influence on the history of the world.
Gunpowder was originally made in private homes, but this habit was so dangerous that its manufacture was limited to special places, such as the Tower of London, in 1461. In the seventeenth century gunpowder became valued as a blasting agent; it was used for rock blasting in Hungary, in the tin mines of Cornwall, and in an engineering project in Switzerland.
A big break-though came in 1846 when the German chemist Schonbein made 'guncotton' by treating cotton fibre with strong nitric and sulfuric acids. The more modern method of making a similar explosive is to use paper or wood-shavings instead of cotton.
A year later, in 1847, an Italian, Ascanio Sobrero, invented one of the most famous of all explosives, nitroglycerine. He dipped glycerine into nitric and sulfuric acids and by this means made an explosive substance that produced 12,000 times its own volume of gas when exploded. However, as nitroglycerine could be set off by rough handling, its use was dangerous and restricted.
But in 1866 the great Swedish scientist Alfred Nobel, after whom the Nobel Prize is called, discovered that if he mixed nitro-glycerine with a sandy substance called kieselguhr its texture became rather like that of cheese and it could be handled with greater ease and safety. This mixture became known as dynamite. He also found that if guncotton was mixed with nitro-glycerine it formed a stiff jelly. This became known as blasting gelatine and is widely used today in quarrying.
Uses of explosives
Apart from their obvious uses in warfare, explosives are of great service to man. They can send rockets soaring upwards into space; they can also help man bore down into the earth in search of coal and other materials. Mining and quarrying would be very laborious jobs without them. It is ironical that though explosives have probably caused more fires than any other substances, they can also be used for extinguishing fires in oil wells. This is done by 'blowing out' the fire with an explosive blast.
The nature of an explosion
The principle of an explosion is very simple and is based on sudden and dramatic expansion. Explosives are substances which are capable of exerting sudden pressure on their surroundings as a result of the rapid conversion of the substance into hot gases. The gases actually occupy the same space as the original substance at the moment of explosion, but the heat of the explosion causes them to expand. The pressure becomes too great for the vessel confining the gases and causes it to burst. Why do we have to use special explosives? Why not anything that can burn? The point about explosives is that they burn at a fantastically high speed, and when they are confined tightly in a restricted space the gases produced by the burning have to burst free. Two of the most important qualities of explosives are:
· They must contain a substance, or mixture of substances, which remains unchanged under ordinary conditions but which undergoes a fast chemical change when the correct thing is done to it, and,
· This change must produce gases whose volume, at the high temperature resulting from the explosion, is much greater than that of the original substance.
If we put these principles into figures we shall obtain some idea of the speed and magnitude of explosions. When the explosive ignites, the gases which it produces can rise in temperature to as much as 11,000°F, which is almost five times as high as the temperature at which steel melts. They are therefore expanding at a tremendous rate and can increase their own volume by about 10,000 times. Something has to give way! When explosives are used for blasting rock they are confined in holes drilled into rock – and it is the rock that give's way. When explosives are used as propellants, it is the shell or missile that gives way by moving forwards or upwards; that is, by being fired.
What are explosives made of?
Because explosives produce a large volume of gas by immediate combustion it is obvious that they must all contain an ingredient which yields the necessary oxygen – without which nothing can burn – and an ingredient which combines with the oxygen. One of the simplest explosives is gunpowder, which is a mixture of potassium nitrate (saltpetre), charcoal, and sulfur. When burnt, it gives off about 4,000 times its own volume of gas. The charcoal and sulfur burn in the oxygen given off by the saltpetre and the action is very rapid. A more powerful explosive – guncotton – which was invented by the German scientist Christian Schonbein in 1846 is made by treating cotton fibre with strong nitric and sulfuric acids. A piece of fiber treated like this would burn swiftly but safely if lit. If struck, however, it would explode with great violence.
Cordite
Cordite is a smokeless, high-explosive shell and bullet propellant containing nitrocellulose (guncotton and nitroglycerin), with petroleum jelly as a stabilizer. Other nitrates were partially substituted for nitroglycerin in World War II to reduce gun-barrel erosion. Manufactured as paste, using acetone as a solvent, cordite is needed into stiff dough and extruded into strands or cords, which give it its name. The cords are then cut into suitable lengths and the acetone evaporated.
Dynamite
Dynamite is a solid, high explosive invented by Alfred Nobel, consisting of nitroglycerin absorbed in an inert material such as kieselguhr or wood pulp. Using nitroglycerin itself, it can be handled safely, not exploding without a detonator. In modern dynamite sodium nitrate replaces about half the nitroglycerin. Gelatin dynamite, or gelignite, contains also some nitrocellulose.
Fulminate
A fulminate is an explosive, a salt of fulminic acid (HONC), especially fulminate of mercury (Hg(ONC)2), which is formed by the action of nitric acid on mercury metal in the presence of alcohol. It is a highly unstable compound used in explosive detonators and blasting caps.
Gunpowder
Gunpowder, also called black powder, is a low explosive, the only one known from its discovery in the West in the thirteenth century until the mid-nineteenth century. It consists of about 75% potassium nitrate (saltpetre) or sodium nitrate, 10% sulfur, and 15% charcoal. When ignited, it expands violently due to the almost instantaneous conversion of the solid ingredients into gases such as carbon dioxide, carbon monoxide, nitrogen, nitrogen oxides, sulfur oxides, and steam. The sudden release of enormous volumes of these gases gives the reaction its explosive force.
Gunpowder was used in fireworks in 10th-century China, as a propellant for firearms from the 14th century in Europe and for blasting since the late seventh century. After about 1900 it was replaced in firearms by smokeless powders such as cordite. It is now used mainly as an igniter, in fuses, and in fireworks.


